Advances in
eISSN: 2572-8490


Case Report Volume 1 Issue 1
1Department of Orthopedic Surgery, Niigata University Medical and Dental Hospital, Japan
2Department of Rehabilitation, Kindai University faculty of Medicine, Japan
3School of Health and Sports Sciences, Mukogawa Women?s University, Japan
4Department of Orthopedic Surgery, Hiroshima University Graduate School of Biomedical Sciences, Japan
Correspondence: Shigeyuki Wakitani, Department of Health and Sports Sciences, Mukogawa Womens University, 6-46 Ikebiraki-cho, Nishinomiya 663-8558, Japan, Tel 81-798-45-9907, Fax 81-798-45-9907
Received: October 10, 2016 | Published: November 7, 2016
Citation: Mera H, Itokazu M, Tamamura Y, Wakitani S. Preservation of independent ambulation 14 years after biological knee reconstruction: a case report. Adv Tissue Eng Regen Med Open Access. 2016;1(1):27-30. DOI: 10.15406/atroa.2016.01.00005
Long-term follow-up showed that biological joint reconstruction, which had been performed by the medical resources at that time, has been reasonable. We report a 26-year-old woman who underwent biological joint reconstruction for a destroyed knee caused by previous treatment for a giant cell tumor of her proximal tibia in 1998. We reconstructed her knee with realignment, including acute shortening and distraction osteogenesis with an Ilizarov external fixator. At follow-up, she could ambulate without pain, and she could run and climb mountains. Magnetic resonance imaging in 2011 showed that the healed tissue had almost the same property as articular cartilage, although it was irregular and thinner. The reconstruction helped preserve her knee function for at least 14 years. Our case shows that some unintentional overlaps such as cell recruitment and the mechanical environment can lead to a good clinical outcome for 14 years.
Keywords: severe joint destruction, biological reconstruction, ilizarov osteogenesis, osteoarthritis, long-term follow-up
In many cases of severe joint disruption, even if the treatment has been thought appropriate and seemed effective in the short-term, there is concern for joint dysfunction and pain caused by the progression of osteoarthritis, including the damage of articular cartilage. Total joint prosthetic replacement is effective and suitable for improving joint function. However, it is desirable to preserve the tissues in such young patients that their life span would be much longer, e.g. more than twice, than of the joint prostheses procedure which might be performed by the medical resources at the time, because of some complications.1–4 Biological joint reconstruction, including realignment osteotomy alone, is therefore more preferable in younger patients, as the success of which will be evident in the long-term. In other word, since it would be revealed only by the long-term follow up whether the biological joint reconstruction, including the treatment of articular cartilage, is successful or not, we need always consider with the best state-of-the art therapy, and incorporate it if necessary, then it is very important to validate the result and consider scientifically about the mechanism. We report our experience with a young female patient with destroyed knee function. Biological knee reconstruction was performed in 1998, and 14 years later, she can ambulate independently, run, and climb mountains without pain.
The patient provided informed consent for the use of her data including previous report5 and ethics approval was waived. A 26-year-old woman presented with severe right knee pain secondary to a pathological fracture of the right proximal tibia caused by a large giant cell tumor. At 52 days post-fracture after the patient had some time to heal, open surgery, including curettage of the tumor, cryosurgery, and packing with bone cement was performed at the previous hospital. Post-operatively, however, she felt continual pain around the knee. Nine months post-operatively, she began to experience uncontrollable increased pain. Radiographic evaluation at 11 months post-operatively showed narrowing of the joint space. She was referred to our hospital.
We first examined the patient in September 1998, 4 years after the primary surgery in 1994. The range of motion of the knee with minimal hydrarthrosis was limited to-10˚ in extension and 120˚ in flexion. Radiographic evaluation showed expansion of the right tibia proximal epiphysis and a varus deformity. The femoro-tibial angle during non-weight-bearing was 185˚. Magnetic resonance imaging (MRI) showed discontinuity in the articular cartilage and/or subchondral bone in the joint surfaces without any signs of tumor recurrence. We performed biological reconstruction in December 1998.
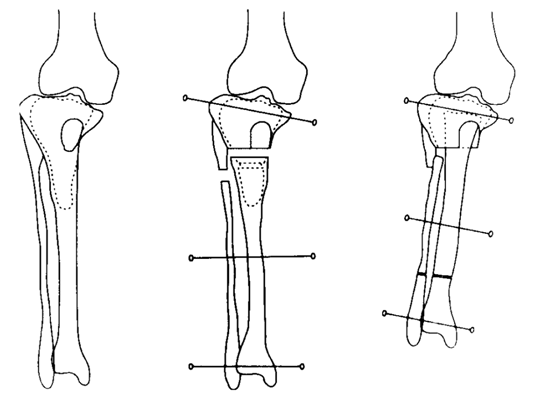
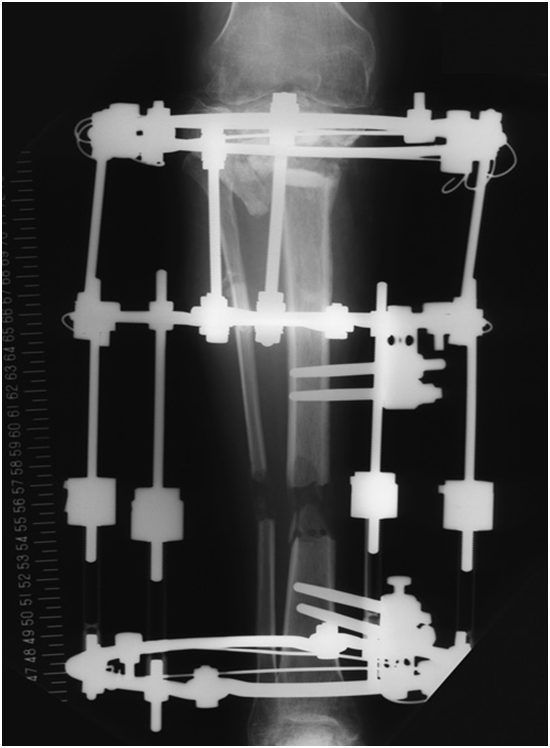
Arthroscopy showed cement protrusion in the intra-articular space, passing through the subchondral bone and articular cartilage in the tibia. The protruded cement had shaved off the articular cartilage on the opposite femoral surfaces. We converted to open surgery to crush and remove the cement. Distraction osteogenesis was performed 1 week post-operatively with the autologous iliac bone graft in the defect combined with the proximal end covered by a glass ceramic, as previously described5 (Figure 1A) (Figure 1B). Distraction was discontinued 4 months later once 54 mm of elongation was performed to resolve the leg length difference. At 12 months post-operatively, bone maturation in the extended division and bone union of the proximal tibia were observed, and all devices were removed. At 17 months after removing the apparatus, the range of motion of the right knee was 0˚ in extension and 110˚ in flexion, and the patient could walk without pain.
In May 2013, almost 14 years and 5 months after reconstruction, she was also able to run, climb stairs, play tennis, and even climb mountains a few hundred meters high. She worked as an officer without any difficulties, except for restricted range of motion of the knee (0˚ in extension and 120˚ in flexion). She had no hydrarthrosis or knee instability. The Knee injury and Osteoarthritis Outcome Score 9 subgroup values were excellent (100 for pain, 89 for symptoms, 100 for activities of daily living, 90 for sports/recreation, and 100 for quality of life).
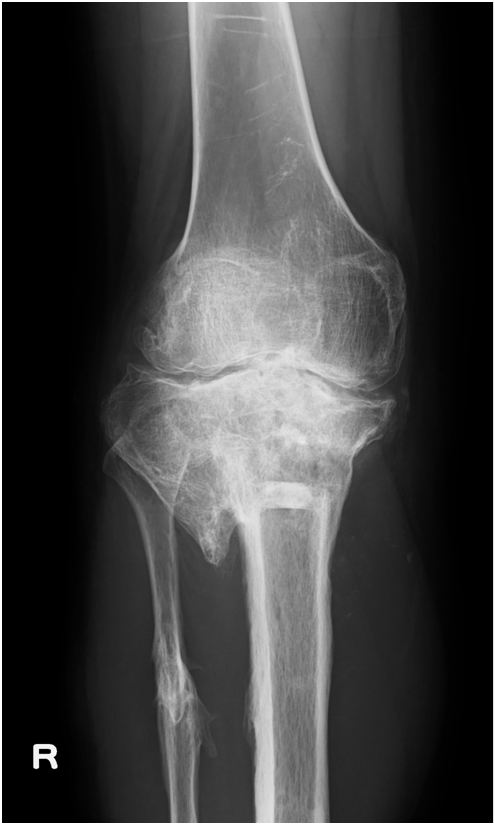
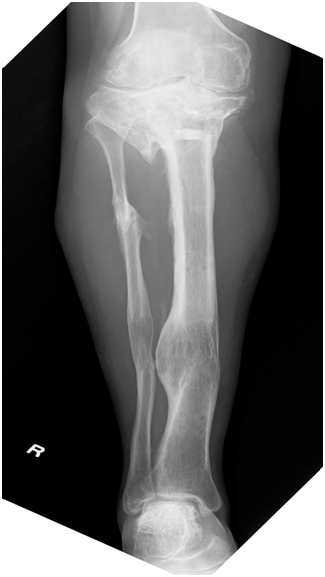
She had undergone radiographic evaluations of the right knee September 14, 2011 (12 years and 9 months post-reconstruction surgery) (Figure 2) and May 24, 2013 (14 years and 5 months post-operatively) (Figure 3), which showed only minor differences between those taken 17 months post-operatively. MRI of the coronal (Figure 4A) and sagittal sections of the lateral condyle (Figure 4B) (Figure 4C) performed September 14, 2011 showed that the tissue had almost the same intensity as the articular cartilage, although it was irregular and thinner. The MRI scans were assessed using the Whole-Organ MRI Scoring system;6 the score was fairly poor (149 points [44.9% of the maximum possible score], including 59 points [70.2%] for cartilage signal and morphology, 10 points [22.2%] for bone abnormality, 6 points [13.3%] for sub articular cysts, 9 points [21.4%] for bone attrition, 51 points [52%] for osteophytes, 12 points [100%] for the meniscus, 1 point [33.3%] for the ligament, and 1 point [33.3%] for synovitis).
Upper T1
Lower T2
A: Coronal section (two slices in the weight-bearing region for each condition).
A small amount of joint fluid was observed. The articular surface was irregular, but the defect in the lateral femoral condyle was repaired with tissue that had almost the same intensity as normal articular cartilage.
Upper T1
Lower T2
B: Two sagittal sections of the lateral compartment.
Upper T1
Lower T2
C: Two sagittal sections of the medical compartment.
Although the articular surface was a little irregular, the joint surfaces of both sides fit well in the widened area, including the meniscus. The tibia bone intensity remained inhomogeneous. The Whole-Organ MRI score was 149 points (44.9% of the maximum possible score).
Figure 4: Magnetic resonance imaging (MRI) examination September 14, 2011.
We performed biological reconstruction of the knee for the present patient in December 1998. We hoped for but did not expect such a good result, and we would have predicted progression to osteoarthritis in the near future.
Articular cartilage has limited repair capacity because of an insufficient supply of progenitor cells and/or growth factors due to their avascularity.7 Many researchers are studying cell transplantation and/or many kinds of growth factors to administer for cartilage repair.8–11 Some trials have been tested in the clinical setting.12–14 The bone marrow stimulation technique includes breaking subchondral bone to facilitate cartilage repair from bone marrow-derived cells. Nevertheless, in cases concomitant with an alignment disorder, it has been demonstrated that following high tibial osteotomy, the cartilage defect can be filled with tissue containing type two collagen, which resembles native hyaline cartilage.15 Long-term success has been indicated, suggesting the existence of cells that may contribute to cartilage repair.
Previously, some studies have investigated the origin of cells that contribute to the tissue repair process, including bone marrow,16 the synovium,17–19 and the articular cartilage itself.20,21 Recently, using green fluorescent protein transgenic and wild type rats of the parabiosis model, as well as the systemic administration of the granulocyte colony-stimulating factor, our group presented evidence of a repair cell population that was supplied by peripheral blood flow22,23 Although we cannot conclude which cells contributed to the repair, there is likely a population of cells that specifically contribute to the healing process at the repair site, creating a stem cell niche.24 Identifying such cells would facilitate targeted therapy to enhance them.
In our case, we had not directly attempted to treat the articular cartilage. Nevertheless, MRI showed that the repair tissue had almost the same property as the articular cartilage, although it was irregular and thinner. Usually, as osteoarthritis progression due to poor repair potential of articular cartilage, the articular cartilage become thinner to deficit and the synovial fluid is accumulated into the space of cartilage defect. Both the medial and lateral meniscus were absent, which is one of the main risk factor of osteoarthritis progression, however, the maintenance of cartilage thickness with same property as the articular cartilage surprised us. Bone marrow edema and synovitis that would cause pain were hardly apparent, which may explain the clinical success of our case. This indicates that the joint can function well without normal anatomy, and the image findings merely show part of the clinical aspects. Additionally, we suggest that our surgery was reasonable; the repair of articular cartilage lasted as long as 14 years after our reconstruction. Because the tibia defects were very large, enough communication between the tibia’s bone marrow and joint space would allow the passage of progenitor cells for cartilage repair, including the femur, although it was untreated. Moreover, there should be an optimum biomechanical environment for cartilage repair. In our case, the tibia plateaus were widened, making the contact area wider, reducing pressure on the articular cartilage and prolonging the repaired articular cartilage matrix. These unintentional overlaps such as cell recruitment and the mechanical environment may explain why cartilage repair in our case was better than we had predicted.
None.
The author declares no conflict of interest.

©2016 Mera, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.