Advances in
eISSN: 2373-6402


Research Article Volume 2 Issue 5
1Vidya Pratishthan School of Biotechnology, India
2Florida Ag Research, Pacific Ag Group, USA
Correspondence: Divya DG, Vidya Pratishthan School of Biotechnology, Vidyanagari, MIDC Bhigwan Road, Baramati-413133 M.S, India
Received: April 14, 2015 | Published: September 2, 2015
Citation: Gabda DD, Aglave B. Studies on bionematicide for the control of plant parasitic nematodes in grape vine & vegetable crops. Adv Plants Agric Res. 2015;2(5):213-219. DOI: 10.15406/apar.2015.02.00064
Root-knot nematodes, ring nematodes, lesion nematodes are the major limiting factor in grapevine and vegetable production in many regions of the world. These nematodes cause significant economic damaged to wide variety of crops. Infected crops showed decrease in yield and quality either directly from root deformation caused by nematodes feeding or indirectly from predisposition to infections by other pathogens that results from nematode penetration into the roots. For the management of these nematodes, botanical plant species of Marigold (Tagetes minuta) was assessed under the field assay and pot assay. The extract of this marigold plant was applied in different concentrations as soil drench in the pots. The different concentrations added in the pots as soil drench were 20%, 40%, 60%, 80% and 100%. The extract of the marigold plant has the active ingredient ‘Alpha-terthienyl’ that plays important role in reducing nematodes and production of healthy plants.
Keywords: botanicals; tagetes plants, root knot nematodes, tomato, cucumber, grapes
Tomato and Cucumber plants (vegetable crops) and Grapes are the one that have maximum damage due to the root knot nematodes infection. Plant parasitic nematodes, are small microscopic roundworms that live in the soil and attack the roots of plants. Several species of Root knot nematodes are Meloidogyne spp., such as M. incognita, M. javanica and M. themasi that have affected the grapevine all over the world.1 The fields in India are also being affected majorly by the nematodes infection. Vegetable crops and Grapevine are majorly infected fields.2
The marigold species most often used for nematode control are Tagetes patula, T. erecta, and T. minuta.3 The key mode by which marigolds suppress plant-parasitic nematodes is through a biochemical interaction known as Allelopathy. Allelopathy is a phenomenon where a plant releases compounds that are toxic to other plants, microorganisms or other organisms, such as nematodes.4 This sulfur-containing compound (‘Alpha-terthienyl’) is abundant in marigold tissues, including roots. It has nematicidal, insecticidal, fungicidal, antiviral, and cytotoxic activities and it is believed to be the main compound responsible for the nematicidal activity of marigold.5 Thus nematodes may be killed either by entering the root system of a marigold plant or contacting soil containing marigold’s bioactive compounds.6
While nematodes do possess a thick cuticle that may provide some protection from predation, this type of defense is easily breached by specialized nematode pathogens. The most common method plant nematodes use to evade predation is by living inside plant tissue or by limiting their mobility in the soil environment.7 Some plant nematodes spend most of their time in the soil (ectoparasites) and others are mostly contained within the plant tissue (endoparasites). Soil populations of most plant parasitic nematodes tend to be highest in September and October after crops have senesced and died. This is the best time of year to sample for nematodes. Sampling in the early fall allows growers time to make decisions on whether to fumigate during the fall or spring or what crop should be planted the following spring. It also allows time to implement an integrated management strategy prior to growing a susceptible crop in that field.8
Taking into consideration all the above facts, the following objectives were undertaken:
Objectives:
Isolation and the identification of the volatile components of the Tagetes minuta
Tagetes minuta is widely cultivated in India. These cultivated plants were then harvested from the field located in Trimbakeshwar. Spacing between the plants was 50cm. The mature plants were collected and dried in the laboratory.
Preparation of extracts and dry powder of botanicals
The extract of the plant products were prepared by grinding the samples with mortar and a pestle. The grinded material was dried and then subjected to the extraction procedure with the help of Soxhlet apparatus. The extraction time required for this procedure is maximum 6hrs. The extract of the plant was purified with the help of GC-MS and the chemical composition of the extract was studied.9 After purification, the extract was poured into the pots containing tomato and cucumber plants and in Grape vineyard.
Standard nematode extraction protocol
The soil sample was collected and weighed (100grams) all the rock and the roots were removed. Sucrose solution (580gm of sucrose in 1000ml of water) was prepared. The soil sample was then mixed with 600ml of water. Allowed to stand for 10minutes and then it was poured through 45/350 mesh sieves. Using a spray bottle the sample was collected into a centrifuge tube. The centrifuge tubes were centrifuged at 1700rpm for 5minutes. Supernatant was discarded and then into the pellet sucrose solution was added and mixed. The tubes were centrifuged at 1000rpm for 30 seconds. Now the supernatant contains the nematodes. Observation of the nematodes was done under 40X objective lens and the population was counted.10
Treatment and experimental design
The experiment had a total of 30 pots in which plantlets of cucumber and tomato were planted. The soil was collected from grape vineyard from Trimbakeshwar that were infected with nematodes, the same soil was used for the growth of the tomato and cucumber (since, same nematodes infect the vegetable crops). The botanical extract treatment was given to each of the pots in successive manner. Each of the 4 pots were applied with the following concentrations 20%, 40%, 60%, 80% and 100% sequentially and 4 pots were left as untreated.
The nematode extraction procedure was carried out before application of each treatment.
Identification of the compound present in the extract obtained from the botanicals (Tagetes plants) was carried out by IR spectroscopy and Gas chromatography-Mass spectrophotometry (Figure 1 & 2).
Gas chromatography
The identification of the essential oil was carried out by Gas chromatography- mass spectrometry (GC-MS).The test was carried out at 250 °C, the oven at initial 500C for 2 minutes increasing 100C per minute till 2000C was reached. The flow of 0.7ul/min at a constant speed of 30cm/s with a 2500C interface was maintained (Figure 3 & Table 1).
Peak # |
R Time |
Area |
Area % |
Name |
1 |
8.825 |
16775364 |
4.54 |
Eugenol |
2 |
12.556 |
46589915 |
12.60 |
Thianthrene |
3 |
13.482 |
7395477 |
2.00 |
9,12-Octadecadienoic acid (Z,Z) |
4 |
14.206 |
14559187 |
3.94 |
2,2:5,2-Terthiophene |
5 |
14.769 |
33636344 |
9.10 |
Thianthrene |
6 |
15.440 |
11955532 |
3.23 |
Hexatriacontane |
7 |
16.160 |
28921424 |
7.82 |
Hexatriacontane |
8 |
17.014 |
52491661 |
14.19 |
Hexatriacontane |
9 |
18.039 |
71110986 |
19.23 |
Hexatriacontane |
10 |
19.285 |
86396882 |
23.36 |
Hexatriacontane |
|
|
369832772 |
100.00 |
|
Table 1 Gas Chromatography
The constituents of volatile oil were identified by calculating their reflective indices under temperature programmed condition. The main constituents of the oil of Tagetes minuta is Eugenol, Thianthrene, Terthiophene (alpha-terthienyl) (Figure 4-7 & Table 2-5).
Plot |
Sting |
Stubby-Root |
Root-knot |
Ptalancheus |
Ring |
Lesion |
Dagger |
1 |
3 |
15 |
24 |
15 |
0 |
6 |
9 |
2 |
5 |
20 |
30 |
15 |
0 |
25 |
0 |
3 |
5 |
10 |
35 |
25 |
0 |
15 |
35 |
4 |
6 |
0 |
36 |
48 |
24 |
24 |
18 |
5 |
7 |
21 |
35 |
42 |
0 |
14 |
14 |
6 |
4 |
40 |
48 |
16 |
0 |
24 |
20 |
Table 2 Precount before product Application
Plot |
Sting |
Stubby-Root |
Root-knot |
Ptalancheus |
Ring |
Lesion |
Dagger |
1 |
2 |
15 |
22 |
15 |
0 |
5 |
9 |
2 |
4 |
14 |
24 |
14 |
0 |
7 |
8 |
3 |
3 |
15 |
24 |
14 |
0 |
6 |
8 |
4 |
2 |
15 |
24 |
15 |
0 |
5 |
9 |
5 |
5 |
19 |
30 |
14 |
0 |
23 |
0 |
6 |
4 |
19 |
29 |
13 |
0 |
24 |
0 |
7 |
3 |
20 |
28 |
12 |
0 |
22 |
0 |
8 |
6 |
19 |
27 |
14 |
0 |
21 |
0 |
9 |
5 |
8 |
34 |
24 |
0 |
12 |
35 |
10 |
5 |
9 |
32 |
23 |
0 |
11 |
33 |
11 |
4 |
8 |
33 |
23 |
0 |
13 |
34 |
12 |
6 |
10 |
34 |
22 |
0 |
10 |
35 |
13 |
5 |
0 |
35 |
45 |
20 |
21 |
18 |
14 |
7 |
0 |
34 |
47 |
22 |
20 |
16 |
15 |
6 |
0 |
36 |
46 |
24 |
22 |
16 |
16 |
4 |
0 |
33 |
44 |
22 |
18 |
16 |
17 |
6 |
21 |
34 |
40 |
0 |
12 |
13 |
18 |
5 |
20 |
35 |
38 |
0 |
11 |
12 |
19 |
5 |
19 |
31 |
40 |
0 |
9 |
12 |
20 |
7 |
19 |
30 |
39 |
0 |
10 |
14 |
21 |
4 |
40 |
46 |
14 |
0 |
20 |
15 |
22 |
3 |
38 |
44 |
13 |
0 |
18 |
16 |
23 |
5 |
36 |
42 |
11 |
0 |
19 |
15 |
24 |
2 |
39 |
44 |
12 |
0 |
18 |
19 |
Table 3 Nematode Population Analysis 15 Days after 1st product application (number of nematodes/100cc of soil)
Plot |
Sting |
Stubby-Root |
Root-knot |
Ptalancheus |
Ring |
Lesion |
Dagger |
1 |
2 |
15 |
22 |
15 |
0 |
5 |
9 |
2 |
4 |
14 |
24 |
14 |
0 |
7 |
8 |
3 |
3 |
15 |
24 |
14 |
0 |
6 |
8 |
4 |
2 |
15 |
23 |
14 |
0 |
5 |
9 |
5 |
5 |
19 |
28 |
12 |
0 |
23 |
0 |
6 |
4 |
19 |
26 |
13 |
0 |
24 |
0 |
7 |
3 |
18 |
26 |
12 |
0 |
22 |
0 |
8 |
6 |
17 |
25 |
13 |
0 |
21 |
0 |
9 |
5 |
6 |
33 |
22 |
0 |
9 |
33 |
10 |
2 |
7 |
30 |
21 |
0 |
7 |
30 |
11 |
1 |
5 |
30 |
22 |
0 |
8 |
31 |
12 |
6 |
6 |
32 |
20 |
0 |
10 |
32 |
13 |
5 |
0 |
32 |
40 |
16 |
17 |
12 |
14 |
2 |
0 |
33 |
42 |
18 |
18 |
13 |
15 |
3 |
0 |
31 |
42 |
15 |
18 |
12 |
16 |
3 |
0 |
30 |
42 |
18 |
13 |
11 |
17 |
4 |
19 |
32 |
38 |
0 |
7 |
9 |
18 |
2 |
17 |
31 |
32 |
0 |
8 |
8 |
19 |
5 |
15 |
28 |
32 |
0 |
4 |
8 |
20 |
2 |
17 |
25 |
31 |
0 |
5 |
7 |
21 |
2 |
30 |
42 |
10 |
0 |
12 |
9 |
22 |
1 |
30 |
41 |
10 |
0 |
11 |
9 |
23 |
3 |
29 |
38 |
6 |
0 |
8 |
9 |
24 |
0 |
31 |
36 |
8 |
0 |
12 |
8 |
Table 4 Nematode Population Analysis 30 days after 1st product application (Numbers of nematodes/100cc of soil)
Plot |
Sting |
Stubby-Root |
Root-knot |
Ptalancheus |
Ring |
Lesion |
Dagger |
1 |
2 |
15 |
22 |
15 |
0 |
5 |
9 |
2 |
4 |
14 |
24 |
14 |
0 |
7 |
8 |
3 |
3 |
15 |
24 |
14 |
0 |
6 |
8 |
4 |
2 |
15 |
23 |
14 |
0 |
5 |
9 |
5 |
5 |
19 |
28 |
12 |
0 |
23 |
0 |
6 |
4 |
19 |
26 |
13 |
0 |
24 |
0 |
7 |
3 |
18 |
26 |
12 |
0 |
22 |
0 |
8 |
6 |
17 |
25 |
13 |
0 |
21 |
0 |
9 |
2 |
2 |
26 |
15 |
0 |
3 |
25 |
10 |
0 |
3 |
25 |
16 |
0 |
4 |
22 |
11 |
0 |
1 |
23 |
15 |
0 |
4 |
23 |
12 |
1 |
2 |
22 |
14 |
0 |
5 |
20 |
13 |
1 |
0 |
27 |
32 |
10 |
10 |
8 |
14 |
0 |
0 |
25 |
34 |
12 |
11 |
9 |
15 |
1 |
0 |
20 |
32 |
11 |
12 |
8 |
16 |
1 |
0 |
22 |
30 |
9 |
7 |
5 |
17 |
2 |
15 |
21 |
30 |
0 |
1 |
4 |
18 |
0 |
14 |
20 |
22 |
0 |
1 |
2 |
19 |
1 |
12 |
20 |
20 |
0 |
0 |
2 |
20 |
0 |
11 |
15 |
22 |
0 |
0 |
3 |
21 |
0 |
22 |
35 |
2 |
0 |
2 |
4 |
22 |
0 |
23 |
33 |
2 |
0 |
1 |
3 |
23 |
1 |
15 |
30 |
0 |
0 |
0 |
3 |
24 |
0 |
12 |
30 |
1 |
0 |
2 |
|
Table 5 Nematode Population Analysis 45 days after first product application
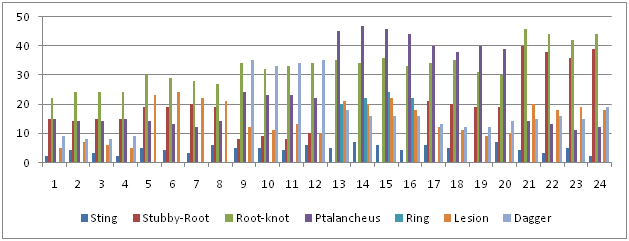
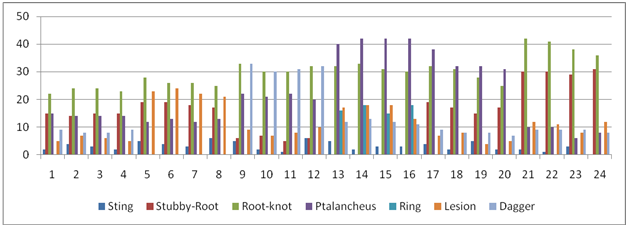
The data recorded from the application of the extract of Tagetes minuta showed that it hampered the growth of the nematodes with the successive addition. The stunted growth was observed in the plants that were left untreated. The plants that were given the treatment showed decreased in the nematode infection. The successive addition of the extract proved harmful for the growth of nematodes (Table 6 & Figure 8).
Tomato |
Cucumber |
|||
|
1 |
2 |
3 |
4 |
1 |
6 |
9 |
8 |
7 |
2 |
6 |
7 |
7 |
7 |
3 |
9 |
4 |
3 |
8 |
4 |
7 |
7 |
3 |
6 |
5 |
5 |
8 |
7 |
5 |
6 |
4 |
6 |
5 |
5 |
7 |
7 |
3 |
2 |
6 |
8 |
6 |
5 |
2 |
5 |
9 |
7 |
8 |
6 |
7 |
10 |
6 |
7 |
8 |
5 |
11 |
7 |
6 |
7 |
6 |
12 |
8 |
7 |
6 |
5 |
13 |
7 |
6 |
7 |
7 |
14 |
6 |
7 |
6 |
7 |
15 |
5 |
6 |
5 |
5 |
16 |
4 |
3 |
7 |
6 |
17 |
7 |
6 |
7 |
7 |
18 |
8 |
7 |
7 |
7 |
19 |
7 |
6 |
6 |
7 |
20 |
9 |
7 |
7 |
6 |
21 |
6 |
6 |
8 |
9 |
22 |
9 |
8 |
7 |
7 |
23 |
6 |
7 |
8 |
8 |
24 |
6 |
7 |
8 |
8 |
Table 6 Root Galling Rate.10
Root galling rate determines the galls’ (swelling) that occurs on the roots due to nematode infestation. The successive addition of the extract of Tagetes minuta hampered the nematodes activity in the soil. (Table 7-9 & Figure 9-11).
Tomato |
Cucumber |
|
1 |
3 |
4 |
2 |
4 |
4 |
3 |
5 |
3 |
4 |
2 |
3 |
5 |
4 |
5 |
6 |
3 |
4 |
7 |
2 |
3 |
8 |
2 |
2 |
9 |
2 |
2 |
10 |
3 |
3 |
11 |
1 |
3 |
12 |
3 |
2 |
13 |
3 |
3 |
14 |
1 |
3 |
15 |
2 |
3 |
16 |
2 |
2 |
17 |
3 |
2 |
18 |
3 |
3 |
19 |
4 |
1 |
20 |
3 |
1 |
21 |
2 |
1 |
22 |
3 |
2 |
23 |
1 |
3 |
24 |
1 |
1 |
Table 7 Sting Root Damage
Tomato |
||
1 |
5 |
6 |
2 |
6 |
6 |
3 |
6 |
5 |
4 |
6 |
6 |
5 |
7 |
7 |
6 |
6 |
6 |
7 |
5 |
5 |
8 |
4 |
4 |
9 |
5 |
5 |
10 |
6 |
5 |
11 |
6 |
6 |
12 |
7 |
6 |
13 |
7 |
7 |
14 |
6 |
5 |
15 |
7 |
7 |
16 |
7 |
6 |
17 |
7 |
7 |
18 |
6 |
7 |
19 |
6 |
5 |
20 |
7 |
6 |
21 |
7 |
8 |
22 |
8 |
8 |
23 |
6 |
7 |
24 |
6 |
7 |
Table 8 Root Vigour Test
Tomato |
||
1 |
6 |
7 |
2 |
6 |
6 |
3 |
5 |
6 |
4 |
5 |
5 |
5 |
6 |
6 |
6 |
6 |
7 |
8 |
7 |
7 |
9 |
6 |
7 |
10 |
5 |
5 |
11 |
6 |
6 |
12 |
6 |
7 |
13 |
7 |
7 |
14 |
6 |
7 |
15 |
6 |
6 |
16 |
5 |
6 |
17 |
7 |
7 |
18 |
7 |
6 |
19 |
7 |
8 |
20 |
7 |
8 |
21 |
6 |
6 |
22 |
6 |
6 |
23 |
8 |
9 |
24 |
9 |
9 |
Table 9 Plant Vigour Test for Tomato
The plant vigour and the root vigour showed the improvement that has resulted in the plants after the application of the Tagetes minutaextract and has improved the plant growth.
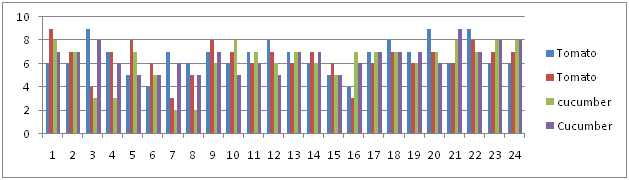
The treatment of tomato and cucumber plants with the botanical extract (Tagetes minuta) increased the germination percentage and establishment of uniform and vigorous seedlings. This is due to direct effect of Tagetes plant product acting as the coat around the seed which prevents the attack of the juveniles by creating unfavorable environment for the nematode activity. These might have influenced the metabolism of germinating seeds rendering the seedlings unfavorable for nematode multiplication as well as increasing the establishment of seedlings.
The nematicidal activity of plant extract is due to the presence of thiophene and alpha-terthienyl in Tagetes minuta that has the same activity against nematodes.6 The extract was applied as a soil drench in successive intervals. The application of the extract into the soil was in different concentration such as 20%, 40%, 60%, 80% and 100%. After 8 days of the plantation of the crop the nematode analysis was done which was untreated and it showed increased number of nematode population.
On 15 day post plantation the first application of the extract was done and the population of nematode was counted. On 30 day post plantation simultaneously the extract was applied to the soil in the same concentration and was analyzed for the counting of nematodes population after 15 days of the first application. The third treatment was given on 45 day post plantation simultaneously analyzing the nematode count of the second treatment and after 15 days of third treatment the analysis of nematode population was done.
The nematode population decreased in the first treatment was just about 1-3%. The nematode population further decreased in the second treatment to 4-7% but the best result was found in the third treatment which was about more than 20%. The negative aspect of soil fumigants and nematicides and the increasing demands for organic procedure makes Tagetes minuta species a potentially valuable alternative to chemical nematicide for nematodes management. Hence, this has provided benefits to farming operations.
None.
The author declares no conflict of interest.

©2015 Gabda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.