
Research Article Volume 9 Issue 1
Rhizoctoniasolani-green beans pathosystem uncover bio control efficacy of Trichoderma spp.
Wafaa Mokhtari,1
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Mohamed Achouri,1 Noureddine Chtaina,2 Hassan Boubaker,3 Abdellah Remah1
1Plant protection department, Institut Agronomique et Vétérinaire Hassan II, Morocco
2Plant Production, Protection and Biotechnology department Institute Agronomique et Vétérinaire Hassan II, Morocco
3Biology department, IbnZohr University, School of sciences, Morocco
Correspondence: Wafaa Mokhtari, Plant protection department, Institute Agronomique et Vétérinaire Hassan II, CHA, BP 121, Agadir, Morocco
Received: December 23, 2018 | Published: January 11, 2019
Citation: Mokhtari W, Achouri M, Chtaina N, et al. Rhizoctoniasolani-green beans pathosystem uncover bio control efficacy of Trichoderma spp. Adv Plants Agric Res. 2019;9(1):96-99. DOI: 10.15406/apar.2019.09.00417
Download PDF
Abstract
Rhizoctoniasolani on green beans has been chosen as one of the best pathosytems to evaluate root rot diseases as well as to determine the effectiveness of potential of the biocontrol agent Trichoderma species (ssp.). In this study we investigate the effective use ofthe pathosystem model; Rhizoctoniasolani-green beans to reveal bio control efficacy of three Trichoderma spp.; T. afro-harzianum, T. reesei and T. guizouhense, isolated from Moroccan soils. In greenhouse conditions, root-dipping approach was involved in revealing bio control potential of localTrichoderma spp. by suppressing root diseases in Rhizoctonia-green beans pathosystem. Interestingly, T. reesei (T9i12) a breaking cellulose succeeded in suppressing disease incidence in root units (DI-RU) = 0.0% in green bean cultivars infected with Rhizoctoniasolani.
Keywords: trichoderma, antagonistic activity, pathosystem, biocontrol potential
Introduction
Rhizoctoniasolani is a soil borne pathogen which is difficult to control because of its sclerotia conserved structure mostly persistent in soil and crop residue and their broad susceptible hosts range. This pathogen may infest post-emerged seedlings after sowing or when transplanting susceptible cultivars. In different place in the world including European, Scandinavian and North African counties, detection and increase of population of virulent Rhizoctonia species (spp). and susceptibility level of cultivars have been determined like that of R. solani in potatoes1,2 and in common beans.3,4 Free pathogen soil-less mix, fumigants control and dry steam treatment program are used to avoid root rots caused bysoil-borne pathogens contamination in greenhouse including Rhizoctonia. However, environmental and health issues attributed to fumigants toxicity has given moving forces to the emergence of bio control beside resistance breeding programs. Therefore, bio control agents (BCA) are more and more applied in integrated plant protection strategies in most of crops-systems.5 Different pathogen-host combinations were chosen as pathosystem models to evaluate biocontrol efficacy in diseases suppression for a BCA to be introduced in crop system. For instance, application of Trichoderma spp. controlled diseases caused by Rhizoctoniain different greenhouse experiments and improved growth and yield parameters of variable cultivars.6,7 MantovanelloLucon et al.,8 applied simultaneously rice seeds colonized with Trichoderma and wheat seeds infected with Rhizoctonia to inoculate cucumber roots’ seedlings. MantovanelloLucon and co-workers found that Trichoderma heals cucumber seedlings damping off in greenhouse conditions. Brewer et al.,9 studied biocontrol efficacy of T. virens and T.harzianum amongst other organisms against Rhizoctonia damping off on potato in greenhouse conditions. They found that both antagonists reduce stem cankers and black scurf on potatoes tubers. This research work focuses on uncovering Trichoderma efficacy to control root diseases in Rhizoctonia-green beans pathosystem in greenhouse conditions.
Materials and methods
Obtaining fungi for test pathogen
Rhizoctonia was isolated from infected roots of olive trees diagnosed with damping off diseases caused by Rhizoctoniasolani. R. solani was isolated from tap roots previously disinfected for one minute in 10% sodium hypochlorite, baited in Potatoes Dextrose Agar (PDA) culture and incubated in 25°C for mycelium to grow. To obtain pure culture Rhizoctonia was sub-cultured in a fresh PDA plates. Rhizoctonia cultures were grown and maintained by subsequent multiplication on PDA (PDA, Difco).
Obtaining Trichoderma isolates
Three Trichoderma isolates were used in this study to evaluate their biocontrol performance in vivo against Rhizoctoniasolani. These isolates were identified in a previous work at the species level as T. afro-harzianum (T8A4), T. guizouhense (T4) and T. reesei (T9i12) respectively.10
Experimental design for trichodermaroot dips treatments in greenhouse conditions
Trichoderma species were tested for their bio control efficacy in Rhizoctonia-green beans. Green beans pathosystem were grown for three to four weeks on 77 peat trays. Seedlings of different cultivars were then transplanted into pots after their inoculation with fungi. Pots where seedlings were transplanted are filled with sterile substrate at 3:1 weight/weight (w/w) peat to sand ratio. Experimental design was organized in four randomized complete blocs with four replicates in each experimental unit. That is, four pots were used in each experimental unit. Four treatments were tested; T1 was designed for treatment of cultivars inoculated with Trichoderma afro-harzianum (T814) and a pathogen, T2 was designed for treatment of cultivars inoculated with Trichodermaguizouhense (T4) and a pathogen and T3 was designed for treatment of cultivars inoculated with Trichodermareesei (T9i12) and a pathogen. TC designed for treatment of cultivars inoculated with Trichoderma afro-harzianum extracted from a commercial product to be compared with other Trichoderma isolates. Two controls were used in this experiment; Tm1 was designed for healthy cultivars (negative controls), Tm2 was designed for cultivars inoculated with pathogen only (positive control).
Inoculum preparation
Rhizoctonia inoculums was prepared using infected corn seeds with Rhizoctonia mycelium. Inoculums type and methods are adopted by Cardoso and Echandi11 with some modifications. In fact, erlen flasks containing 50 corn seeds and distilled water were sterilized at 120°C for 1 hour in autoclave and sterilization procedure was repeated after 24 hours. Excess of water was discarded and each flask was inoculated with 4 big square pieces of a growing culture of R. solani on PDA. Infected corn seeds were incubated at 24°C for 25 days and each flask were shaken every 4 days to allow uniform seed colonization by Rhizoctoniasolani.11
Root-dipping of green beans seedlings with Trichoderma spp and artificial infection with Rhizoctonia
Roots of seedlings were dipped with fungal suspensions at transplanting. Green beans’ roots were respectively dipped first into a suspension of 2.106 and 108 conidia ml-1 of different Trichoderma spp. for 15 to 20 minutes (min).9 Pathogens inoculation of beans’ roots was performed using two Rhizoctonia infected corn seeds per pot as inoculum. Corn seeds were placed near to beans’ roots at the moment of transplanting.
Disease evaluation
Diseases assessment was estimated by measuring Disease Incidence (DI) of the pathogen as reported by previous studies.12 Disease Incidence (DI) was measured as the percentage of number of plant units showing typical symptoms of each related pathogen. Therefore, DI percentage was calculated as shown in the equation (2) given by Benson and Baker (1974):
Disease Incidence(DI)=number of infected plantstotal number of plants*100(2)
Disease incidence was measured in above ground units of the plant (DI-AU) and root units (DI-RU) three months after Trichoderma spp. treatments. In this study we evaluate disease incidence at two level measuring disease incidence in aerial plant units (DI-AU) and root units (DI-RU) at the end of each experiment. Symptoms and signs recovered from roots, crown, stem and leaves were recorded. In addition, plant parameters like roots dry weight (RDW) and plant dry weight (PDW), plant height (PH), leaves surface area (LSA) and green bean pods number (Pods N°) were also measured.13,14
Statistical analysis
Diseases incidence measurement data were subjected to variance analysis for reliable estimation of the amount of disease. Greenhouse experimentation out puts; DI, root and plant dry weight, leaves surface area and green bean pods number were suggested to analysis of variance to determine differences between four treatments (T1, T2, T3, TC) and to compare with the controls Tm1 and Tm2.
Results
Suppression of Rhizoctoniasolani by Trichoderma root dips treatments in green beans
It can be inferred from DI-AU and DI-RU results that Rhizoctonia disease can be efficiently controlled by Trichoderma spp. treatments. Significant decrease of disease response in root unit has been observed in different in Trichoderma spp. root dipped green beans cultivars (p=0.000). Disease incidence recorded in commercial Trichoderma in TC and T. afro-harzianum in T1 treatments and found to not exceed 30% incidence in green beans roots. While, root disease incidence in T. reesei (T2) treatment was DI-RU= 0.0% similarly compared to Tm1 as indicated in
figure 1.Specific symptoms were observed in positive controls Tm2 investigated in pathogenicity test of Rhizoctonia. Disease caused by Rhizoctonia was assessed by root and stem rots and lesions, red dashes on the surface of green beans stem designated as cankers in the infected green beans. Lesions were not seen in any of treated green beans cultivars. Root dry weight recorded in green beans treated with T. reesei (T2) was RDW=4.3 grams (g) and was fairly compared to RDW of healthy green beans in Tm1 with RDW= 4.1g. T1 and TC treatments showed lower root weight with RDW=1.0 g and 1.2 g respectively as shown in
figure 2. Green beans in T2 and Tm1 contain the highest numbers of pods which is above 30, then, T1, TC and T3 had between 20 to 25 pods, where, Tm2 contains only 15 pods. RDW and PH data suggest that Trichoderma spp. treatments efficiently maintain and improve plant fitness even when infected with Rhizoctonia. In fact, the highest the RDW and PH of green beans, the lowest disease responses in aerial and roots units of green beans and the symptomless plants expression were recorded. In the present study, T. reesei in T2 treatment successfully suppressed Rhizoctonia disease responses (see
Figure 3). In fact, T. reesei has succeeded in suppressing stem cankers in green beans. Also, other treatment; T. afro-harzianumin T1 treatment, T. guizouhensein T3 and commercial Trichoderma in TC have noticeably reduced stem cankers.
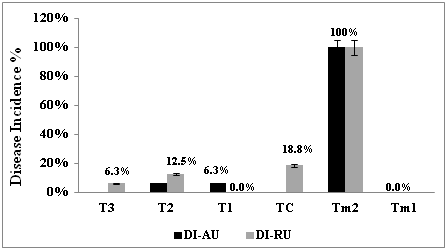
Figure 1 Disease incidence in (%) in Rhizoctoniasolani-green beans pathosystem and bio control effect of Trichoderma spp.; T. afro-harzianum in treatment T1, T. reesei in treatment T2, T. guizouhense in treatment T3 and TC Trichoderma extracted from commercial product used as reference.
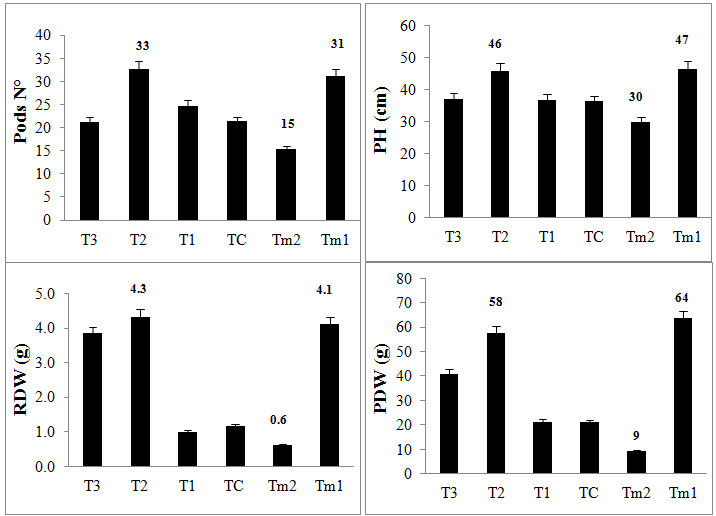
Figure 2 Effect of Trichoderma treatment on plant development parameters in Rhizoctonia-green beans pathosystem. Plant development parameters measured were; Pods Number (Pod N°), Plant Height (PH), Root Dry Weight (RDW) and Plant Dry Weight (PDW).

Figure 3 T. reesei in treatment T2 suppress cankers lesions in stem and rots in roots compared to controls Tm2.
Discussion
Trichoderma root-dips using localTrichoderma spp. have reduced considerably Rhizoctonia disease responses in different cultivars. T. afro-harzianumand T. reesei gave promising results on reducing symptoms caused by this pathogen. Brewer et al.,
9 have demonstrated the efficiency of T. harzianum and T. virens in suppressing disease response caused by Rhizoctoniasolani in potatoes. Their results showed less stem cankers and black scurf in potatoes tubers when treated with T. harzianum. Interestingly, Trichodermareesei in T2 treatment was able to suppress Rhizoctoniasolani disease response on green beans. It was able to suppress stem cankers and lesions and roots rots in beans cultivars. Trichodermareesei have been reported to serve as a workhorse for industrial cellulosic enzymes production. However, it’s important to mention that Trichodermareesei under strain code: QM 6a has been employed as an antagonist against Rhizoctoniasolani.
15 Trichoderma treatments effect on disease suppression was well correlated with their effect on plant development parameters like plant height and root dry weight of green beans. Therefore, it may be inferred that different Trichoderma treatments reliably affect green beans growth. It seems that root-dipping revealed to be effective carrier of Trichoderma spores towards site of infection in roots. That is, Trichoderma efficacy on root diseases suppression demonstrated the good establishment of the antagonists on the site of inoculation. Root-dipping approach has been investigated in our previous study using Verticillium-eggplant pathosystem and showed the power of this treatment to reveal Trichoderma bio control performance.
16–18 It can be inferred that root-dipping applied at seedling transplantation can effectively be used as root drench treatment to control root disease of Rhizoctoniasolani in green beans in greenhouse.
Acknowledgements
None.
Discloser statement
No potential conflict of interest was reported by the authors.
References
- Elbakali AM, Lilja A, Hantula J, et al. Identification of Spanishisolates of Rhizoctoniasolani frompotato by anastomosisgrouping, ITS-RFLP and RAMS-fingerprinting. Phytopathologia Mediterranea. 2003;42:167–176.
- Lehtonen MJ, Somervuo P, Valkonen JPT. Infection with Rhizoctonia solani Induces Defense Genes and Systemic Resistance in Potato Sprouts Grown Without Light. Phytopathology. 2008;98(11):1190–1198.
- Das S, Shah FA, Butter RC, et al. Genetic variability and pathogenicity of Rhizoctoniasolaniassociatedwith black scurf of potato in New Zealand. Plant Pathology. 2013;63(3):651–666.
- Mayo S, Gutiérrez S, Malmierca MG, et al. Influence of Rhizoctoniasolani and Trichoderma spp. ingrowth of bean (Phaseolusvulgaris L.) and in the induction of plant defense-relatedgenes. Front Plant Sci. 2015;6:685.
- Aleandri MP, Chilosi G, Bruni N, et al. Use of nursery potting mixes amended with local Trichoderma strains with multiple complementary mechanisms to control soil-borne diseases. Crop Protection. 2014;67:269–278.
- Howell CR, Hanson LE, Stipanovic RD, et al. Induction of terpenoid synthesis in cotton roots and control of Rhizoctoniasolaniby seed treatment with Trichodermavirens. Phytopathology. 2000;90(3):248–252.
- Singh P, Raja RB. Biological synthesis and characterization of silver nanoparticles using the fungus Trichodermaharzianum. Asian Journal of Experimental Biological Sciences. 2011;2:600–605.
- MantovanelloLucon CM, Mitsue Koike C, Ishida Ishikawa A, et al. Bioprospection of Trichodermaspp. isolates to control Rhizoctoniasolanion cucumber seedling production. Pesqui Agropecu Bras. 2009;44(3):225–232.
- Brewer MT, Larkin RP. Efficacy of several potential biocontrol organisms against Rhizoctoniasolani on potato. Crop Protection. 2005;24(11):939–950.
- Mokhtari W, Achouri M, Remah A, et al. Verticilliumdahliae-Eggplant as the pathosystem Model to Reveal Biocontrol Potential of three Trichodermaspp in Greenhouse Conditions. Atlas Journal of Biology. 2018;6:417–421.
- Cardoso J, Echandi E. Biological control of Rhizoctonia root rot of snap bean with binucleateRhizoctonia-like fungi. Plant Disease Journal. 1987;71:167–170.
- Kranz J. Measuring Plant Disease. In: Kranz J, Rotem J, editors. Experimental Techniques in Plant Disease Epidemiology. Berlin. Springer. 1998. 35–50 p.
- Benson DM, Baker R. Epidemiology of Rhizoctoniasolani pre-emergence damping-off of radish: influence of pentachloro-nitrobenzene. Phytopathology. 1974;64:38–40.
- Campbell CL, Neher DA. Estimating disease severity and incidence. In: Campbell CL, Benson DM editors. Epidemiology and Management of Root Diseases. New York. Springer. 1994. 117–147 p.
- Atanasova L, Crom S, Gruber S, et al. Comparative transcriptomics reveals different strategies of Trichodermamycoparasitism. BMC Genomics. 2013;14:121.
- Mokhtari W, Chtaina N, Halmschlager E, et al. Potential antagonism of some Trichoderma strains isolated from Moroccan soil against three phytopathogenic fungi of great economic importance. Revue Marocaine des Sciences Agronomiques et Vétérinaires. 2017;5:248–254.
- Baek MJ, Greer CA, Webster RK. Et al. Population Structure of Rhizoctoniaoryzae-sativae in CaliforniaRice Fields. Patcharavipa Chaijuckam, The American Phytopathological Society. 2010;100(5):502–510.
- vanBruggen AHC, Whalen CH, Arneson PA. Effect of Inoculum Level of Rhizoctoniasolani on emergence, Plant development, and Yield of Dry Beans. The American Phytopathological Society. 1986;76: 869–873.

©2019 Mokhtari, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.



