Advances in
eISSN: 2373-6402


Research Article Volume 4 Issue 1
Department of Post-graduate Studies, K R College Mathura, India
Correspondence: Ravi Sharma, Department of Post graduate studies, KR College Mathura, India
Received: March 30, 2016 | Published: July 5, 2016
Citation: Sharma R. Indole-acetic acid oxidase enzyme activity in three wheat cultivars under salt stress conditions at the early seedling stage. Adv Plants Agric Res. 2016;4(1):215-222. DOI: 10.15406/apar.2016.04.00123
Indole-acetic acid enzyme activity in three wheat cultivars differing in their salt response was studied at the early seedling stage. It was found that the IAA oxidase enzyme activity gradually decreased with increasing EC levels in both the salt-sensitive cultivar IWP-72 and moderately salt-tolerant cultivar Sharbati sonora while in the tolerant cultivar HD-2160 the enzyme activity increased till 96 hours up to 12 EC while at 120hours at all EC levels the activity decreased. The magnitude was, however, higher in the salt-sensitive cultivar. The IAA oxidase activity increased with advancement in seedling age but the degree of increase varied with the cultivars. Whereas in the tolerant and moderately tolerant cultivars a sharp increase was observed at 72 and 96hours which later declined at 120hours, on the other hand, in the sensitive cultivar it increased from 24hours reaching a peak level at 120hours. Also from the observations it is noticed that IAA oxidase enzyme activity was affected more in the salt-sensitive cultivar (IWP-72) as compared with the moderately salt-tolerant (Sharbati sonora) and the salt-tolerant cultivar (HD-2160).
Keywords: wheat (triticum aestivum L), NaCl salt stress, Salt-tolerant cultivar (HD-2160), moderatety salt-tolerant cultivar (Sharbati sonora), salt-sensitive cultivar (IWP-72), IAA oxidase enzyme activity
Plant germination and growth is regulated by the balance of plant hormones such as IAA, GA, CK etc.,1-5 and any disturbance in the balance of plant hormones retard the plant growth in which IAA plays a predominant role whose level, of course, is maintained endogenously by the enzyme activity of IAA oxidase.4-6 Several workers7-22 have shown genotypic variation among the same species of crop plants in relation to salt stress. In the present work an attempt has been made to find out a relationship existing between salt tolerance and sensitivity with IAA oxidase enzyme activity, if any, in three cultivars of wheat, salt-tolerant (HD-2160), moderately salt-tolerant (Sharbati sonora) and salt-sensitive (IWP-72). Thus, the objective of the study is to correlate relationship between salt tolerance and IAA oxidase enzyme activity.
Forty two wheat cultivars (Triticum aestivum L) procured from Wheat Directorate, Cummings Laboratory, Division of Genetics and Plant Breeding, Indian Agricultural Research Institute, New Delhi and Chandra Sekhar Azad University of Agriculture and Technology, Kanpur (UP), India22 were subjected to screening for salt resistance by Garrad’s Technique23 modified by Sarin et al.,24 and Sharma8,22 and as per method of Sheoran et al.,25 The shoot and root lengths of seedlings were recorded at definite interval of 24hours using test tubes of uniform size (30ml capacity) fitted with rolls of filter paper folded at the top into a cone to support the seeds. The tubes were filled to one-third part with the test solutions so that the solution might not come in direct contact with the growing roots and to ensure that the salt solution being supplied to the roots through capillary action of the filter paper. Distilled water (represented the mean loss of water from the blanks) was added to each test tube after every 24hours of interval in order to maintain salt concentration near the target levels throughout the germination period. The seeds were initially sterilized with 0.1 percent mercuric chloride (HgCl2) solution and later washed thoroughly with distilled water. Three seeds per tube were then transferred to the edge of the filter paper cone and were allowed to grow between the filter paper roll and the wall of the test tube in dark growth chamber at 25±20C. Fifteen replicates (five tubes each having three seeds) were maintained for each treatment including the controls (half-strength Hoagland solution grown). Observations on the influence of salinity levels at 4, 8, 12 and 16 EC dsm-1 of salt solution and the controls on the total length of shoot and root at early seedling stage were recorded at 24hour intervals from 48 hours after sowing up to the end of 120hours under green safe light. The relative tolerance of different cultivars was evaluated on the basis of the percentage reduction in shoot growth at 12 EC.22
Three selected cultivars viz., salt-tolerant (HD-2160), moderately salt-tolerant (Sharbati Sonora) and salt-sensitive (IWP-72) from the entire screening were studied for IAA oxidase enzyme activity under salt-stressed conditions. The seeds were germinated and seedlings grown in sterilized petridishes on moistened filter paper kept in a dark growth chamber at 25±20C. The ‘treated sets’ contained the salt (NaCl) solution at different EC (4, 8, 12 and 16) levels and the ‘controls’ half-strength Hoagland solution. Samples were collected at 24hour intervals from 48hours after sowing up to the end of 120hours. The seedlings were raised according to the method described previously4,26 and IAA oxidase enzyme activity was determined according to Rabin et al.,27 technique as described by Witham et al.,28 and Sharma et al.,4 The enzyme assay was conducted in cold room. One g of the seedlings was homogenized with 10 ml of cold distilled water in a chilled mortar. The slurry was passed through a layer of cheese cloth. The filtrate thus obtained was centrifuged at 10,000rpm for 15minutes. To the supernatant excess of acetone was added so that the final acetone concentration was brought to 40 percent by volume. It was centrifuged at 1,000 rpm for 15minutes. After discarding the supernatant the pellet of precipitate was re-suspended in 4ml of 0.2M phosphate citrate buffer (pH 5.6)* containing 0.5ml10-4M MnCl2 and 0.5ml 10-3M 2,4 dichlorophenol. The precipitate was shaken vigorously to dissolve it and the resulting solution was used as enzyme preparation. The reaction mixture containing 2 ml of the enzyme preparation, 7ml of 0.2M phosphate citrate buffer (pH 5.6) and 1ml of indole-acetic acid (200µg/ml) was incubated for one hour at 300C. After incubation residual IAA was estimated by taking 1ml of the reaction mixture and adding 4ml Salkowski reagent and was left to stand for 30minutes at room temperature. Pink colour of IAA was read at 540nm against a reagent blank. The results are expressed as µg IAA destroyed per g fresh wt per hr.
*Phosphate citrate buffer (0.2M pH 5.6): was prepared from-A. 19.21g citric acid dissolved in 1000ml of distilled water; B. 53.65g sodium hydrogen phosphate (Na2HPO4.7H2O) dissolved in 1000 ml of distilled water. Working Solution was prepared by mixing 21.0ml of solution A with 29.0 ml of B diluted to a total of 100ml and adjusted pH to 5.6. All parameters were analyzed by ‘Analysis of Variance’ (ANOVA) method as given by Panse et al.,29
Wheat seedling growth
In the screening of the forty two wheat cultivars for salinity tolerance at the early seedling stage shoot and root lengths under varying salinity levels (0, 4, 8, 12 and 16 dsm-1) induced by NaCl were observed. In the ANOVA analysis all the main effects viz., variety (V), treatment (T) and seedling age (D) and their interactions (V x D; V x T; D x T and V x D x T) were found to be highly significant at 0.01 probability with significant differences noticed in the shoot and root growths of all the cultivars studied.22 With this, relative shoot and root growths of the cultivars showed that shoot growth was affected more as compared with root growth under salt stress. On the other hand, all the cultivars showed an increase in shoot and root growths with seedling age. It was evident that the different cultivars exhibited marked differences in their early seedling growth with increasing age of the seedling and that with advancement of seedling age the effect of salt declined and that, in general, tolerance to salinity increased (Figure 1). As indicated earlier22 only 11 cultivars showed less than 60 percent reduction in shoot growth while majority of the 31 cultivars had more than 60 percent reduction at 16 EC. This is in contrast with root growth where almost a reverse trend was noticed, i.e., out of the 42 cultivars only 15 showed more than 60 percent reduction at 16 EC whereas 27 had less than 60 percent reduction (Table 1). The observations recorded clearly indicated that the shoot is more sensitive to salt stress than the root and that shoot growth is a better index of relative salt tolerance of different cultivars of the same species at early seedling stage with this also 12 EC salinity level was found to be a critical level for majority of the cultivars. Thus, on the basis of the percent reduction in shoot growth at 12 EC level over respective control all the cultivars were categorized into three groups, viz., salt-tolerant, moderately salt-tolerant and salt-sensitive, showing less than 40%, 40–60% and more than 60% reduction respectively (Table 2).
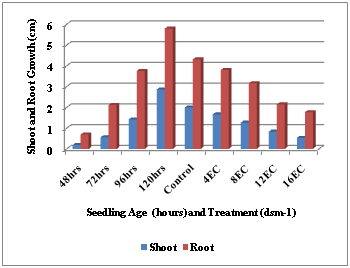
Figure 1 Relative Shoot and Root Growths of Certain Wheat (Triticum aestivum L) Cultivars Under Salt Stress at the Early Seedling Stage.22
Further, the different rates of shoot growth of the three groups as affected by increasing level of salinity showed a gradual decline in both the salt tolerant and moderately salt–tolerant cultivars. On the other hand, the salt–sensitive cultivars had a sharp decline in growth with increasing salt concentrations. With this the relative comparison of seedling growth between three wheat cultivars studied indicated better performance of HD–2160 (salt-tolerant) at almost all levels of salinity when compared with controls. It showed highest tolerance to salinity (i.e., 85.219 percent), IWP-72 (salt-sensitive) showing highest inhibition (i.e., only 7.818 percent) and Sharbati sonora (moderately salt-tolerant) showing (48.574) percent shoot growth at 12 EC over control (Figure 2).
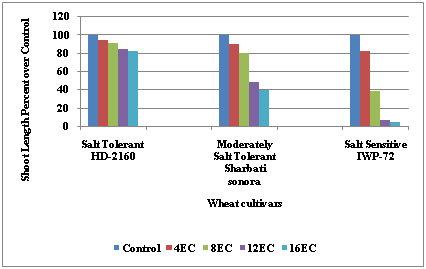
Figure 2 Relative Salt Tolerance of Three Groups (salt-tolerant, moderately salt-tolerant and salt-sensitive) Wheat (Triticum aestivum L) Cultivars Under Salt Stress at the Early Seedling Stage (Data Expressed as Percent Over Control).
S.No |
Cultivar |
Shoot Growth |
Root Growth |
||||||
4EC |
8EC |
12EC |
16EC |
4EC |
8EC |
12EC |
16EC |
||
1 |
HD-2236 |
113.561** |
56.847 |
11.491 |
5.599 |
111.150** |
84.029 |
22.589 |
12.905 |
2 |
WL-410 |
113.259** |
74.804 |
43.746 |
18.731 |
114.330** |
92.463 |
75.146 |
47.188 |
3 |
*Sharbati sonora |
90.591 |
80.876 |
48.574 |
40.3 |
98.463 |
95.181 |
80.079 |
59.912 |
4 |
Moti |
95.025 |
66.374 |
11.423 |
9.995 |
87.94 |
70.758 |
37.162 |
29.576 |
5 |
Sonalika |
86.523 |
70.728 |
48.179 |
43.217 |
95.222 |
89.47 |
80.958 |
66.694 |
6 |
*HD-2160 |
95.135 |
91.113 |
85.219 |
82.6 |
94.623 |
83.76 |
77.584 |
71.188 |
7 |
HD-2135 |
73.548 |
52.043 |
35.555 |
9.892 |
81.956 |
65.602 |
43.14 |
28.569 |
8 |
IWP-503 |
80.606 |
59.617 |
29.346 |
13.939 |
93.087 |
80.71 |
60.972 |
31.834 |
9 |
HS-43 |
92.583 |
64.833 |
43.948 |
29.261 |
92.614 |
75.133 |
59.131 |
40.818 |
10 |
UP-262 |
78.452 |
59.844 |
28.956 |
8.15 |
85.716 |
66.802 |
44.781 |
26.168 |
11 |
HD-2177 |
79.002 |
58.31 |
28.527 |
6.716 |
109.224** |
89.42 |
55.588 |
36.919 |
12 |
WG-1559 |
83.288 |
25.802 |
20.454 |
9.358 |
83.98 |
20.319 |
15.742 |
6.588 |
13 |
HD-2267 |
89.577 |
49.142 |
11.873 |
8.245 |
79.032 |
28.403 |
16.921 |
2.87 |
14 |
*IWP-72 |
82.921 |
39.094 |
7.818 |
5.144 |
84.874 |
48.701 |
14.736 |
5.826 |
15 |
HD-2282 |
95.6 |
89.584 |
57.47 |
35.213 |
95.272 |
93.368 |
65.889 |
56.434 |
16 |
WL-711 |
92.973 |
89.329 |
71.437 |
46.128 |
97.498 |
88.607 |
74.932 |
54.35 |
17 |
Raj-1482 |
96.668 |
71.829 |
38.573 |
32.378 |
96.239 |
78.587 |
48.426 |
44.231 |
18 |
HD-2260 |
77.059 |
72.661 |
70.284 |
22.894 |
92.593 |
89.221 |
77.579 |
40.201 |
19 |
WH-246 |
92.266 |
59.304 |
43.644 |
33.929 |
114.221** |
77.774 |
56.468 |
49.96 |
20 |
WL-2200 |
62.53 |
112.53 |
46.654 |
35.279 |
84.951 |
105.439** |
71.072 |
60.244 |
21 |
K-7634 |
96.841 |
92.545 |
80.353 |
52.179 |
85.271 |
90.808 |
80.487 |
59.321 |
22 |
Raj-1556 |
81.372 |
66.86 |
52.875 |
44.063 |
81.503 |
74.521 |
59.752 |
55.695 |
23 |
UP-154 |
90.714 |
75.952 |
60.714 |
49.523 |
91.303 |
86.454 |
75.083 |
68.645 |
24 |
HD-1977 |
83.333 |
54.973 |
43.01 |
40.456 |
88.177 |
54.75 |
48.064 |
41.449 |
25 |
WG-1558 |
94.865 |
71.393 |
43.276 |
35.207 |
130.566** |
120.546** |
65.282 |
43.319 |
26 |
HD-2204 |
65.638 |
76.945 |
42.878 |
34.948 |
77.622 |
91.666 |
62.237 |
55.594 |
27 |
WL-1531 |
90.816 |
84.081 |
71.02 |
33.061 |
91.401 |
79.841 |
66.907 |
40.895 |
28 |
K-7631 |
73.214 |
68.75 |
56.25 |
47.321 |
76.935 |
72.955 |
63.467 |
58.887 |
29 |
Raj-1409 |
69.243 |
46.546 |
38.98 |
17.708 |
81.585 |
59.013 |
44.596 |
27.941 |
30 |
Raj-1493 |
75.996 |
64.855 |
50.815 |
26.721 |
73.242 |
67.101 |
60.822 |
37.093 |
31 |
Raj-1494 |
44.152 |
39.379 |
28.353 |
11.217 |
58.24 |
42.093 |
35.408 |
22.251 |
32 |
WL-903 |
75.341 |
68.003 |
59.726 |
38.822 |
93.159 |
85.064 |
69.72 |
57.263 |
33 |
UP-171 |
82.361 |
60.073 |
17.195 |
14.612 |
80.992 |
59.594 |
38.173 |
28.072 |
34 |
HD-2275 |
83.125 |
64.034 |
25.738 |
17.329 |
79.938 |
64.834 |
43.078 |
37.768 |
35 |
HD-1593 |
30.959 |
45.903 |
27.746 |
14.944 |
44.547 |
56.477 |
34.855 |
20.465 |
36 |
HD-2252 |
106.760** |
75.329 |
65.759 |
35.381 |
109.373** |
76.631 |
71.436 |
42.741 |
37 |
HP-1303 |
86.174 |
56.969 |
54.166 |
39.09 |
79.185 |
68.89 |
67.294 |
42.038 |
38 |
UP-115 |
85.802 |
72.619 |
66.535 |
40.169 |
89.683 |
81.821 |
58.298 |
42.637 |
39 |
HD-1980 |
94.002 |
60.403 |
54.406 |
44.369 |
93.379 |
58.454 |
52.941 |
40.599 |
40 |
CC-464 |
57.12 |
51.009 |
46.866 |
24.08 |
72.834 |
69.253 |
61.864 |
47.878 |
41 |
HD-2009 |
88.962 |
60.902 |
57.321 |
39.097 |
83.056 |
69.413 |
63.068 |
50.406 |
42 |
Kharchia |
69.192 |
49.329 |
43.035 |
30.542 |
90.201 |
70.451 |
65.195 |
55.269 |
CD at 5% P = 0.064 S.Em. ± 0.023 |
CD at 5% P = 0.351 S.Em. ± 0.126 |
||||||||
Table 1 Shoot and Root Growth of Forty two Wheat Cultivars at Different Salinity Levels
(Data expressed as percent over control) (Cultivars with asterisk* used in the present study)
(Cultivars with ** showed stimulation observed in growth at moderate levels of salinity)
It is evident from the Tables 3 and 4 that the IAA oxidase enzyme activity gradually decreased with increasing salt stress created by NaCl in both the salt-sensitive cultivar IWP-72 and moderately salt-tolerant cultivar Sharbati sonora while in the tolerant cultivar (HD-2160) the enzyme activity increased till 96 hours up to 12 (dsm-1) EC salinity level, but at 120 hours at all EC levels the activity decreased, although, the magnitude of the enzyme activity was higher in the sensitive cultivar IWP-72. The IAA oxidase activity increased with advancement in seedling age with varying degree in the three cultivars. Whereas in the tolerant and moderately tolerant cultivars a sharp increase was observed at 72 and 96 hours declining later at 120 hours, on the other hand, in the sensitive cultivar enzyme activity increased from the very beginning at 48 hours reaching peak level at 120 hours. Also from the observations it is noticed that IAA oxidase enzyme activity was affected more in the salt-sensitive cultivar (IWP-72) as compared with the moderately salt-tolerant (Sharbati sonora) and the salt-tolerant cultivar (HD-2160). As shown in the Table 5 in the three cultivars there is direct correlation found in the shoot growth, root growth and IAA oxidase enzyme activity as with increasing salt concentration there is decrease in root and shoot growth and also the enzyme activity. On the other hand, with seedling age as the root and shoot growth increased the IAA oxidase activity also increased but till 96 hours and at 120 hours it slightly declined (Table 6).
Group I Salt-Tolerant (Less than 40% Reduction) |
Group II Moderately Salt-Tolerant (40 – 60% Reduction) |
Group III Salt-Sensitive (More than 60% Reduction) |
||||
Wheat Cultivars |
1. HD-2160* |
85.219 |
1. WL-903 |
59.726 |
1. Raj-1409 |
38.98 |
2. K-7634 |
80.353 |
2. HD-2282 |
57.47 |
2. Raj-1482 |
38.573 |
|
3. WL-711 |
71.437 |
3. HD-2009 |
57.321 |
3. HD-2135 |
35.555 |
|
4. WL-1531 |
71.02 |
4. K-7631 |
56.25 |
4. IWP-503 |
29.346 |
|
5. HD-2260 |
70.284 |
5. HD-1980 |
54.406 |
5. UP-262 |
28.956 |
|
6. UP-115 |
66.535 |
6. HP-1303 |
54.166 |
6. HD-2177 |
28.527 |
|
7. HD-2252 |
65.759 |
7. Raj-1556 |
52.875 |
7. Raj-1494 |
28.353 |
|
8. UP-154 |
60.714 |
8. Raj-1493 |
50.815 |
8. HD-1593 |
27.746 |
|
9. Sharbati Sonora* |
48.574 |
9. HD-2275 |
25.738 |
|||
10. Sonalika |
48.179 |
10. WG-1559 |
20.454 |
|||
11. CC-464 |
46.866 |
11. UP-171 |
17.195 |
|||
12.WL-2200 |
46.654 |
12. HD-2267 |
11.873 |
|||
13. HS-43 |
43.948 |
13. HD-2236 |
11.491 |
|||
14. WL-410 |
43.746 |
14. Moti |
11.423 |
|||
15. WH-246 |
43.644 |
15. IWP-72* |
7.818 |
|||
16. WG-1558 |
43.276 |
|||||
17. Kharchia |
43.035 |
|||||
18. HD-1977 |
43.01 |
|||||
19. HD-2204 |
42.878 |
|||||
Table 2 Relative Tolerance of Certain Cultivars of Wheat Based on the Percent Reduction in Shoot Growth at 12 EC (dsm-1) Salinity Level
Variety |
Treatment NaCl Conc. (EC dsm-1) |
Shoot Length (cm) |
Root Length (cm) |
IAA oxidase activity (µg IAA destroyed/g fresh wt/hr) |
|||||||||
Duration (hours) |
Duration (hours) |
Duration (hours) |
|||||||||||
48hrs |
72hrs |
96hrs |
120hrs |
48hrs |
72hrs |
96hrs |
120hrs |
48hrs |
72hrs |
96hrs |
120hrs |
||
C1 HD-2160 (Salt-tolerant) |
Control |
0.27 |
0.49 |
0.95 |
2.56 |
1.14 |
2.3 |
3.7 |
7.36 |
224 |
262 |
262 |
248 |
4 EC |
0.27 |
0.41 |
0.85 |
2.53 |
0.96 |
2.18 |
3.68 |
6.9 |
240 |
248 |
312 |
220 |
|
8 EC |
0.25 |
0.41 |
0.84 |
2.39 |
0.78 |
2.05 |
3.57 |
5.75 |
281 |
292 |
292 |
232 |
|
12 EC |
0.23 |
0.39 |
0.83 |
2.19 |
0.68 |
1.89 |
3.19 |
5.49 |
262 |
278 |
286 |
224 |
|
16 EC |
0.23 |
0.37 |
0.78 |
2.15 |
0.62 |
1.5 |
2.9 |
5.3 |
232 |
250 |
256 |
198 |
|
C2 SHARBATI SONORA (Mod. Salt-tolerant) |
Control |
0.95 |
1.45 |
3.96 |
6.67 |
1.75 |
4.33 |
6.87 |
8.73 |
282 |
296 |
320 |
232 |
4 EC |
0.76 |
1.36 |
3.26 |
6.43 |
1.45 |
4.31 |
6.3 |
8.66 |
262 |
276 |
282 |
198 |
|
8 EC |
0.51 |
1.02 |
2.9 |
6.12 |
1.43 |
4.26 |
6.04 |
8.32 |
242 |
242 |
256 |
195 |
|
12 EC |
0.08 |
0.61 |
1.98 |
3.68 |
0.69 |
3.11 |
5.75 |
7.28 |
198 |
213 |
232 |
122 |
|
16 EC |
0.06 |
0.61 |
1.74 |
2.85 |
0.55 |
2.88 |
3.41 |
5.79 |
120 |
140 |
146 |
127 |
|
C3 IWP-72 (Salt-sensitive) |
Control |
0.38 |
1.05 |
2.86 |
5.42 |
1 |
2.65 |
4.66 |
8.14 |
240 |
290 |
320 |
356 |
4 EC |
0.3 |
0.94 |
2.2 |
4.62 |
0.71 |
2.5 |
4.45 |
6.3 |
202 |
218 |
230 |
290 |
|
8 EC |
0.2 |
0.36 |
1.12 |
2.12 |
0.41 |
1.65 |
2.54 |
3.4 |
188 |
226 |
240 |
272 |
|
12 EC |
0.12 |
0.16 |
0.2 |
0.28 |
0.1 |
0.25 |
0.65 |
1.42 |
122 |
134 |
218 |
218 |
|
16 EC |
0.1 |
0.12 |
0.12 |
0.16 |
0.04 |
0.12 |
0.22 |
0.58 |
90 |
120 |
204 |
218 |
|
SEm |
±0.053 |
±0.065 |
±9.332 |
||||||||||
Table 3 Effect of Different Levels of Salt Stress (NaCl) Exposure on Shoot Length, Root Length and IAA Oxidase Enzyme Activity in three Wheat Cultivars
Variety |
Treatment NaCl Conc. (EC dsm-1) |
Shoot Length (cm) |
Root Length (cm) |
IAA oxidase activity(µg IAA destroyed/g fresh wt/hr) |
|||||||||
Duration (hours) |
Duration (hours) |
Duration (hours) |
|||||||||||
48hrs |
72hrs |
96hrs |
120hrs |
48hrs |
72hrs |
96hrs |
120hrs |
48hrs |
72hrs |
96hrs |
120hrs |
||
C1 |
Control |
0.27 |
0.49 |
0.95 |
2.56 |
1.14 |
2.3 |
3.7 |
7.36 |
224 |
262 |
262 |
248 |
HD-2160 |
4 EC |
0.27 |
0.41 |
0.85 |
2.53 |
0.96 |
2.18 |
3.68 |
6.9 |
240 |
248 |
312 |
220 |
(Salt-tolerant) |
8 EC |
0.25 |
0.41 |
0.84 |
2.39 |
0.78 |
2.05 |
3.57 |
5.75 |
281 |
292 |
292 |
232 |
12 EC |
0.23 |
0.39 |
0.83 |
2.19 |
0.68 |
1.89 |
3.19 |
5.49 |
262 |
278 |
286 |
224 |
|
16 EC |
0.23 |
0.37 |
0.78 |
2.15 |
0.62 |
1.5 |
2.9 |
5.3 |
232 |
250 |
256 |
198 |
|
C2 SHARBATI SONORA |
Control |
0.95 |
1.45 |
3.96 |
6.67 |
1.75 |
4.33 |
6.87 |
8.73 |
282 |
296 |
320 |
232 |
(Mod. Salt-tolerant) |
4 EC |
0.76 |
1.36 |
3.26 |
6.43 |
1.45 |
4.31 |
6.3 |
8.66 |
262 |
276 |
282 |
198 |
8 EC |
0.51 |
1.02 |
2.9 |
6.12 |
1.43 |
4.26 |
6.04 |
8.32 |
242 |
242 |
256 |
195 |
|
12 EC |
0.08 |
0.61 |
1.98 |
3.68 |
0.69 |
3.11 |
5.75 |
7.28 |
198 |
213 |
232 |
122 |
|
16 EC |
0.06 |
0.61 |
1.74 |
2.85 |
0.55 |
2.88 |
3.41 |
5.79 |
120 |
140 |
146 |
127 |
|
C3 |
Control |
0.38 |
1.05 |
2.86 |
5.42 |
1 |
2.65 |
4.66 |
8.14 |
240 |
290 |
320 |
356 |
IWP-72 |
4 EC |
0.3 |
0.94 |
2.2 |
4.62 |
0.71 |
2.5 |
4.45 |
6.3 |
202 |
218 |
230 |
290 |
(Salt-sensitive) |
8 EC |
0.2 |
0.36 |
1.12 |
2.12 |
0.41 |
1.65 |
2.54 |
3.4 |
188 |
226 |
240 |
272 |
12 EC |
0.12 |
0.16 |
0.2 |
0.28 |
0.1 |
0.25 |
0.65 |
1.42 |
122 |
134 |
218 |
218 |
|
16 EC |
0.1 |
0.12 |
0.12 |
0.16 |
0.04 |
0.12 |
0.22 |
0.58 |
90 |
120 |
204 |
218 |
|
SEm |
±0.053 |
±0.065 |
±9.332 |
||||||||||
Table 4 Effect of Different Levels of Salt Stress (NaCl) Exposure on Shoot Length, Root Length and IAA Oxidase Enzyme Activity in Three Wheat Cultivars (Data Expressed as Percent Over Control)
Treatment NaCl (EC dsm-1) |
Shoot Length (cm) |
Root Length (cm) |
IAA oxidase activity (µg IAA destroyed/g fresh wt/hr) |
Control |
2.251 |
4.386 |
277.667 |
4 EC |
1.994 |
4.033 |
248.167 |
8 EC |
1.52 |
3.35 |
246.528 |
12 EC |
0.896 |
2.542 |
208.917 |
16 EC |
0.774 |
1.992 |
175.111 |
SEM |
± 0.015 |
± 0.019 |
± 2.694 |
Table 5 Effect of Different Levels of Salt Stress (NaCl) Exposure on Shoot Length, Root Length and IAA Oxidase Enzyme Activity in Three Wheat Cultivars (TREATMENT)
SD: Standard Deviation; BMI: Body Mass Index; WC: Waist Circumference; AC: Abdominal Circumference; HC: Hip Circumference; RER: Respiratory Exchange Ratio; HR: Hear Rate.
Duration (hours) |
Shoot Length (cm) |
Root Length (cm) |
IAA oxidase activity (µg IAA destroyed/g fresh wt/hr) |
48 hrs |
0.314 |
0.821 |
212.333 |
72 hrs |
0.65 |
2.399 |
232.333 |
96 hrs |
1.639 |
3.862 |
257.067 |
120 hrs |
3.345 |
5.961 |
223.378 |
SEM |
± 0.014 |
± 0.017 |
± 2.409 |
Table 6 Effect of Different Levels of Salt Stress (NaCl) Exposure on Shoot Length, Root Length and IAA Oxidase Enzyme Activity in Three Wheat Cultivars (DURATION)
One of the most common effects of salinity is stunting of growth often without any other sign of damage. This and other modifications of growth habit have suggested that growth hormones may be involved in the responses of plants to salinity.30 Germinating seeds have been found to be rich sources of hormones such as IAA, GA, Kinins and some inhibitors also.31-35 It has been observed that the salinity inhibits seedling growth and that the inhibition is directly correlated to the increase in salinity. To further understand this growth inhibition the pattern of hormonal regulation has been studied as IAA and GA contents along with the enzymes which regulate these systems.5,8,7,36,37 Chen3 suggested that these endogenous levels of plant hormones play a predominant role in growth regulation which depends upon the balance between the growth promoters [e.g., GA, Kinins etc.,] and the inhibitors [e.g., ABA or in some instances IAA]. It had been observed that with increasing salinity IAA oxidase activity increased in the tolerant cultivar, while it decreased in the sensitive cultivar. Also IAA oxidase activity increased with seedling age and reached a peak at 96 hours and later it declined, in the three cultivars. Verga & Balint36 have also reported similar behavior of IAA oxidase activity during early seedling growth. Pilet & Gastner38 have observed that one of the mechanisms controlling growth via endogenous auxins is through enzymatic oxidations. IAA oxidase is regarded to oxidize the IAA, thereby controlling the endogenous level in the seedlings. Interestingly it was observed in the tolerant cultivar while the auxin-like promoters were highest at 24 hours the IAA oxidase activity was lowest (Table 6). On the other hand, the sensitive cultivar showed peak auxin levels at 48 hours it also exhibited a higher level of IAA oxidase. Therefore, it is likely that the reduction in auxin-like promoters observed in the present investigation as a result of salt stress is mediated through the enzyme IAA oxidase. Goyal & Baijal26 reported that IAA oxidase enzyme activity of the different cultivars of the same species is regulated by the specific gene in each cultivar, therefore, a genetic diversity was found in the growth behavior of the three cultivars differing in salt tolerance as well as in IAA oxidase enzyme activity. From our laboratory workers7-22 have shown genotypic variation among the same species of crop plants in relation to salt stress as shown in the present work.
From the results presented, it appears that the growth under salt stress condition is mediated by endogenous IAA level at different state regulated by IAA oxidase enzyme system indirectly affecting metabolism. Thus, resulting in differential growth response of the three wheat cultivars viz., salt-tolerant (HD-2160), moderately salt-tolerant (Sharbati sonora) and salt-sensitive (IWP-72) in relation to salt stress and that the tolerance of a cultivar depends mainly on the endogenous IAA at the effective level.
Author is indebted to Dr. BD Baijal (Retired Professor Plant Physiology Department of Botany Agra College, Agra) for expert comments and to the Principal KR College, Mathura, India for providing necessary facilities.
The author declares no conflict of interest.

©2016 Sharma. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.