Advances in
eISSN: 2373-6402


Research Article Volume 9 Issue 1
1Department of Agronomy, Faculty of Agricultural Science, Ladoke Akintola University of Technology Ogbomoso, Nigeria
2Department of Biology, Federal University of Technology Akure, Nigeria
Correspondence: Emmanuel Ayodeji, Oguntola, Department of Biology, Federal University of Technology Akure, Nigeria
Received: December 03, 2018 | Published: January 11, 2019
Citation: Fagbemigun OV, Oguntola EA. Effect of organomineral nitrogen starter fertilizer on the growth and yield of groundnut (Arachis hypogeal L.). Adv Plants Agric Res. 2019;9(1):86-94. DOI: 10.15406/apar.2019.09.00416
The need for starter nitrogen in production of groundnut have been established, therefore the need to test different nitrogen sources. A field experiment was carried out at the Teaching and Research farm at Ladoke Akintola University of Technology, Ogbomoso in the year 2012 to determine the optimum rate of starter nitrogen on growth and yield of groundnut (Arachis hypogeal L.) using organomineral fertilizer (Alesinloye Asejere fertilizer). Two cultivars; Samnut 23 and Campala which were sown together on two different dates (02/06/2012 and 16/06/2012) with the application of five nitrogen rates which are 0, 5, 10, 15 and 20 kgN/ha altogether translate to give 20 treatment combinations. The treatments were replicated three times and were a split-split-plot experiment laid out in random complete block design. The responses of groundnut to treatment were assessed starting from four weeks after planting (WAP) and at two weeks interval. Data were collected on plant height, number of nodules, number of leaves, fresh weight of root, stem and leaves, number of pods, total dry matter, length of leaves and breadth of leaves were taken and subjected to the analysis of variance and means were separated using Duncan multiple range test (DMRT) at 5% level of probability. Analysis of results revealed that Campala had the higher number of leaves production capacity (93.80 per plant at 10 WAP) compared with Samnut 23 (50.53 per plant at 10 WAP). However Samnut 23 produced higher pod yield (16.50 per plant at 10 WAP) and Campala (12.10 per plant at 10 WAP). The result also revealed that Campala is better for foliage production especially as fodder or soil management purposes. Early planting and application of 10gN/ha produced significantly higher yield.
Groundnut (Arachis hypogeal L.) originated in South America (Bolivia and adjoining countries) and is now grown throughout the tropical and warm temperate regions of the world. This crop was grown widely by native people of the New World at the time of European expansion in t he sixteenth century and was subsequently taken to Europe, Africa, Asia and the Pacific Islands. It is an important oil and food crop which is currently grown on approximately 42 million acres worldwide. It is the third major oil seed of the world next to soybean (Glycine max) and cotton (Gossypium sp.).1 Groundnut has only occasionally been grown in northern states due to its warm temperate requirement.2,3 The leading producing states in Nigeria includes: Niger, Jigawa, Kebbi, Kano, Katsina, Adamawa, Bornu, Yobe, Taraba, Zamfara, Sokoto, Kaduna, Taraba, Gombe and Plateau (Abulu, 1978). Groundnut contains 25–32% protein, 21% carbohydrates, 42–52% oil and 4.26 water.4 All parts of the groundnut plant can be used. The groundnut grown for human consumption has several uses as whole, seeds or is processed to make peanut butter, oil and other products.4 It is consumed chiefly as roasted seeds or peanut butter. Non food products such as soaps, medicines, cosmetics, and lubricants can be made from peanuts. The vines with leaves are excellent high protein hay for horses and ruminant livestock. The pods and shells serve as high fibre roughage in livestock feed, fuel (Fire place “logs”), mulch, and is used in manufacturing particle board or fertilizer. The cake residue from oil processing is used as an animal feed and as a soil fertilizer. Groundnut shells are used in the manufacture of plastic, wallboard, abrasives, fuel, cellulose (used in rayon and paper) and mucilage (glue). Groundnut plants serve the purpose of covering as cover crops for lands exposed to the soil erosion and it fixes nitrogen into the soil through the nodules that developed at the roots. A crop rotation of groundnut–cereal– cereal helps in efficient utilization and reduces soil born diseases and nematodes. It also helps to reduce the incidence of weeds (Henning et al., 1982; Alison 1981) Temperature is the major limiting factor for peanut yield in northern states since a minimum of 3,000 growing degree – days (with a base of 50oC) are required for proper growth and development.2 Groundnut requires a light-coloured and textured soil with good drainage and low moderately low amounts of organic matter for its optimum performance. Such soil is preferred since it is usually loose and friable, permitting easier penetration of roots and pegs, better percolation of rainfall, and easier harvesting. Well-drained soils provide proper aeration for the roots and nitrifying bacteria that are necessary for proper mineral nutrition of the plant. Medium to heavy soils or those with a high clay content leads to loss of pods during harvesting. Groundnut grows best in slightly acidic soils with a pH of 6.0 to 6.5, but a range of 5.5 to 7.0 is acceptable.5 Groundnut responds well to residual soil fertility from previous crops in the rotation, but usually has a low response to fertilizer in soil with medium to high fertility levels. It fixes atmospheric nitrogen with the help of rhizobium in the root nodules. This helps to partially fulfill its nitrogen requirement. However, it takes about 25–30 days to develop root nodules. Therefore, some available nitrogen is required in the early stages for plant growth. An application of 10kgN/ha at the time of sowing is recommended for soils with moderate to low nitrogen content as ammonium sulphate. The requirement for phosphorus in nodulating legumes higher compared with non-nodulating crops. If available soil phosphorus is less than 15kg/ha, there is need to apply phosphatic fertilizer. Single superphosphate is recommended because it contains phosphorus (7.0%), calcium (19.5%), and sulphate (12.5%) that are required by groundnut.6 A potassium application may not be required unless there is less than 125kg/ha available in the soil. Groundnut also requires high calcium during the pod filling stage. Although, the nitrogen requirement is usually met by the atmospheric nitrogen it fixes into the soil through the initial growing stage when the root nodules have not developed. Organomineral nitrogen starter fertilizer is a form of fertilizer from both biological and synthetic source which is applied to the soil before or immediately after planting to enhance the early formation of root nodules in leguminous plants so as to fix nitrogen into the soil. Hence, the objectives of this study were to: determine the effect of organomineral nitrogen starter in the growth and yield of groundnut and to infer recommendations based on the data obtained from the results of the study.
History of groundnut
The domesticated groundnut is an amphidiploids or allototraploid, meaning that it has two sets of chromosomes from two different species, thought to be Arachis duranensis and Arachis ipaensis. These likely combined in the wild to form the tetraploid species. Arachis monticola, which gave rise to the domesticated groundnut. This domestication might have taken place in Paraguay or Bolivia, where the wildest strains grow today. Many pre-Columbian cultures, such as the Moche, depicted groundnuts in their arts. Archeologists have dated the oldest specimens to about 7,600 years, found in Peru. Cultivation spread as far as Mesomerica where the Spanish conquistadors found the Halcacaluat (Nahualt = ‘groundnut’, whence Mexican Spanish, Cacahuate and French, Cacahuete) being offered for sale in the market place of Tenochtitian (Mexico City). The plant was later spread worldwide by European traders.
Botanical description and growth habits
Groundnuts (Arachis hypogeal L.) is a self-pollinating, indeterminate, annual, herbaceous legume. Natural cross pollination occurs at rates than 1% to greater than 6% due to a typical flower or action of bees.7 The fruit is a pod with one to five seeds that develops underground with a needle-like structure called a peg an elongated ovarian structure. Groundnut emergence is intermediate between the epigeal (hypocotyls elongates and cotyledon emerge above ground as in soybean) and hypogeal (cotyledons remain below ground as in maize) types. The hypocotyls elongates but usually stops before cotyledons emerge. Leaves are alternate and pinnate with four leaflets (two pairs of leaflets per leaf. The groundnut plant can be erect or prostrate (6 to 24 inches or more) with a well developed taproot and many lateral roots and nodules. Plants develop three major stems, i.e., two stems from the cotyledonary auxiliary buds equal in size to the central stem during early growth. Bright yellow flowers with both male and female parts are located on inflorescences resembling spikes in the axils of leaves. One to several flowers may be present at 34 each node are usually more abundant at lower nodes. The first flowers appear at 4 to 6 weeks after planting and maximum flower production occurs 6 to 10 weeks after planting. Eight to 14 days after pollination aerial pegs will grow 2 to 3 inches into the soil and then turn to a horizontal orientation to mature into a groundnut pod. Pods reach maximum size after 2 to 3 weeks in the soil, maximum oil content in 6 to 7 weeks, and maximum protein content after 5 to 8 weeks. The groundnut crop matures after 7 to 9 weeks in the soil, which is indicated by maximum levels of protein, oil, dry matter and presence of darkened veining and brown splotching inside the pod. Groundnuts usually require a minimum of 100 to 150 days from planting to maturity depending on the variety planted. Flowering continues over a long period and pods are in all stages of development at harvest. Pegs will eventually rot in the soil (25% after 12 weeks in the soil) and the resulting loose pods are lost during the harvest. Since the pod wall is needed to protect the seed as it is moved through the various markets form producer to processor or consumer, yields and farm prices are based on a pod rather than seed basis.
Environmental requirement of groundnut
Soil for groundnut production should be a light-coloured, light textured with good drainage, and moderately low amounts of organic matter. Such soil is preferred since it is usually loose and friable, permitting easier penetration of roots pegs, better percolation of rainfall, and easier harvesting. Light-coloured soils reduce staining of pods which ensures greater eye appeal when crop is used for unshelled nuts. Well drained soils provide proper aeration for the roots and nitrifying bacteria that are necessary for proper mineral nutrition of the plant. Medium to heavy soils or those with high clay content should also be avoided due to excessive less of pods when harvesting groundnuts. Organic matter should be maintained at a level of 1 to 2% to improve water-holding capacity of the soil and supply plant nutrients. Groundnut grows best in slightly acidic soils with pit of 6.0 to 6.5, but a range of 5.5 to 7.0 is acceptable. Saline soils are not suitable since groundnut has a very low salt tolerance.5 As already mentioned in the introduction, temperature is the major limiting factor for groundnut yield in northern states with a base of 50oC is required or proper growth and development.2 A groundnut crop will not reach optimum maturity for a marketable yield to justify commercial production in areas with fewer heat units during growing season. Little if any growth and development occur at temperatures below 56oC.8 and 68oC is reported to be optimal by Kething9 Poor stand is perhaps the most common cause of low yields. To obtain a full stand, use undamaged seed with intact seed coats and treat shelled seed with an approved seed protectants prior to planting. Planting seeds rather than pods allows for easier machine planting and more uniform stands. Robinson2 reported higher yields when seed was used because planting pods delayed emergence due to slower absorption of moisture into the shells.
Fertilizer recommendation for groundnut
Groundnut will not grow well or fix nitrogen in acidic or infertile soils. The soil pH should be between 5.3 and 6.5; the crop should not be grown on soils of pH above 7.5. Groundnut can benefit from residual fertility and in general, no additional fertilizer is needed if the crop is grown on well-managed land previously treated with a balanced fertilizer. Most soils in rain-fed agriculture contain too little phosphorus, so in order to ensure good plant establishment, high yield, and good seed quality, apply single superphosphate before or at sowing. It will supply the crop with the phosphate, sulphate and calcium that are essential for crop growth and development. The fertilizer recommendation is more important for large seeded Virginia types than for the small-seeded Spanish types. The large-seeded have thick shells and require more fertilizer input to facilitate normal seed development while small-seeded types have thin shells and need less fertilizer application.
The role of N-P-K in legume and other crops production
Groundnut fixes atmospheric nitrogen with the help of rhizobium in the root nodules. This helps to partially fulfill its nitrogen requirement. However, it takes about 25–30 days to develop root nodules.6 Therefore, some available nitrogen is required in the early stages for plant growth. An application of 10kgN/ha as ammonium sulphate at the time of sowing is recommended for soils with moderate to low nitrogen content.6 Nitrogen’s deficiency in groundnut plants result in younger leaves becoming lighter green than normal. In severe cases, the entire leaf becomes pale yellow. Stems are thin and elongated. In mature plants, older leaves fall. Growth is stunted, and the stem becomes reddish poor pod and kernel development. The application of digested cake, organomineral fertilizers and urea to winter wheat resulted in significantly different crop responses with responses with respect to the control (no fertilizer added). However, crop yield and weight of 1000 grains were not significantly affected by fertilizer type, nitrogen application rate, or the interaction between them. Also, the application of Urea-N at high rate (300kgN/ha) resulted in residual mineral–N which is thought to be responsible for sustaining a marginally higher yield in the third cut compared to organomineral fertilizers when used at the same nitrogen-rate. However, mineralization of organomineral fertilizer-nitrogen between the second and the third cuts contributed to significantly reduce the differences in grass yield between urea and organomineral fertilizers amended soils late in the season. Faster N release from urea compared with organomineral fertilizers results in significantly larger amounts of plant available N which results in enhanced nitrogen up-tae and increased grass yield.10 Phosphorus is the key element needed to establish root growth as a seed begins to sprout. The benefits of using a starter fertilizer with an ideal amount of phosphorus are many. The phosphorus in the soil aids new roots by causing them to grow quickly. It also enhances a plant’s ability to flower. There is debate about the need for phosphorus if soil tests indicate adequate levels are already present, but most starter fertilizer contains a higher concentration of phosphorus to make it readily accessible to new roots.6 The requirement for phosphorus in nodulating legumes is higher compared with non-nodulating crops. If available soil phosphorus is less than 15kg/ha, there is need to apply phosphatic fertilizer. Single superphosphate is recommended because it contains phosphorus (7.0%), calcium (19.5%), and sulphur (12.5%) that are required by groundnut.6 Its deficiency in groundnut plant results in stunted plants. Leaf size is reduced. Initially affected plants becomes bluish, late a dull and dark green. Older leaves turn orange yellow. Later the entire leaf becomes brown and finally drops. Potassium is the third ingredient in the N-P-K designation. It is used to stave off the effects of cold weather and fight off diseases. It also contributes to the process of photosynthesis. Potassium application may not be required unless there is less than 125kgK/ha available in the soil. Its deficiency in groundnut plants results in marginal yellowing, and scorching at maturity, leaf margins curl upward and the leaf dies.
Planting date and depth
Groundnuts were traditionally planted in April before tomato spotted wilt virus (TSWV). This disease has pushed the planting window later and the recommended date is now May 11-25. Groundnuts can do well planted through the first week of June and will suffer yield loss planted later than this.11 Planting in early June was originally favoured in Minnesota due to the warm temperature required for optimal growth of groundnut. However, planting in early May required 9 more days to emerge and had a slower development than a crop planted in June. However, the planting in early May flowered earlier which allowed more pods to reach maturity before frost.12 Groundnuts may be planted deeper than any of the other row crops. It will emerge from 5-7cm deep or deeper. However, like other seed that are high in oil content, seed should be planted into moisture for rapid germination.11
Harvesting and storage of groundnut
The optimum time for harvesting is when most pods have a veined surface, seed coats are coloured, and 75% of pods show darkening on the inner surface of the hull. Harvesting in northern areas should begin after the first killing, first if soil moisture is at a level suitable for cultivation since wet soil sticks to pods. Harvesting usually starts with clipping or coultering. Rotary mowers remove up to half of the tog growth is too great for efficient harvesting. A killing frost may make this step necessary, since most of the leaves may have already fallen off the plant.8 The two most important operations in handling groundnut after harvest are cleaning and drying to safe moisture content (5to10%). Pods should be kept dry and protected against infestation from insects or rodents, as well as from loss of natural colour and flavor, and prevention of the development of off-flavours and rancidity. Artificial drying of wet or semi dry groundnut shout start immediately after combining to prevent mold growth and aflatoxin formation. Presence of aflatoxin is a concern in groundnut production states with warmer climates. Unheated air may be used for drying causes physical and biochemical changes that can be harmful or beneficial to flavor and quality. Robinson2 reported that groundnut maintains moisture content of about 7% at a relative humidity of 65to 70%. Groundnut is usually stored in the form of unshelled nuts. Seven to eight month storage is usually required for groundnut used as seed and those intended for food uses can be stored until the start of next harvesting season (Emery and Hexam, 1969). Seed retained longer when stored in the pod than when shelled. Seed stored with 5% moisture content lost viability more slowly than seed with 8% moisture, but relative humidity must be less than 50% maintain such a low moisture level.8
Disease of groundnut and their control
Groundnut rosette diseases
It is caused by virus. Mode of transmission is through piercing and sucking insects known as aphids. Major observable symptoms of groundnut rosette diseases are stunting/shortening of internodes and leaf petiole; yellowing of groundnut leaves; reduction of leaf area and curling/rolling and twisting of groundnut leaves (Olabiyi, 2004) Groundnut rosette diseases can be controlled by uprooting and burning all infected groundnut plants, earlier planting with close spacing is recommended, planting of resistance varieties and control of insect vector (aphid) with the appropriate chemical insecticides (Olabiyi, 2004)
Leaf spot (Tikka) disease
It is a fungus disease. The causal organisms are Cercospora arachidicola and Cercospora personata. Major symptom of the disease on groundnut is the appearance of leaf spots. It is an air-borne disease. To control leaf spot disease of groundnut, farmers are advised to do seed-dressing (seed treatment) before planting groundnuts; and planting of resistance varities (olabiyi, 2004)
Mouldy seeds disease
It is a fungus disease. Even though it is soil borne, it attracts onlt the groundnut seeds. The causal organism is Aspergillus flavus. The production of aflatoxin which is toxic to man and livestock gives groundnut seeds a foul smell and bad taste. Groundnut seeds becomes mouldy. To control mouldy seed disease in groundnut, resistance and mould free seeds should be planted, seed treatment with appropriate fungicides before planting are required (Olabiyi, 2004)
Location of the experimental site
The experiment was carried out at Ladoke Akintola University of Technology (LAUTECH), teaching and Research Farm in Ogbomoso, Oyo State, Nigeria. Ogbomoso is on the longitude 4o101E and Latitude 8o101N. The climate is mostly influenced by North East trade wind, which is characterized with cold wind, with a drying effect starting from November till March. Ogbomoso climate is also affected by Southwest trade wind, which is warm and moist from April to October. The temperature of the year ranges from 28oC to 33oC with relative humidity of about 74% all year round except in January, when the dry wind blow from the North. The annual rainfall is over 1000mm. The physical and chemical properties of experimental sire are as shown in the table below:
Experimental design and treatments
The 2 x 2 x5 factorial experiment was a split-split plot experiment laid out in randomized complete block design with three replicates. Each of the replicates has 20 treatment plots in it which gives a total of 60 treatment plots. Each treatment plot size was 1.5mx2m and the spacing between one treatment and other on the same replicate was 1m while the spacing between the replicate was 1m. Planting was 25cm x 50cm and the planting depth of the groundnut was 5cm. The treatments were three: two groundnut cultivars (samnut 23 and campala), two planting dates (02/06/12 and 16/06/12) and five rates of organomineral fertilizers (0 knN/ha, 5 kgN/ha, 10kgN/ha, 15kgN/ha and 20kgN/ha). The plot layout is shown in Figure 1.
Field preparation and management
The experimental site was cleared by using hoes and cutlasses. The debris were removed and burnt on a spot outside the experimental site after which the site was leveled manually with the use of hoe. The whole experimental plot was fenced round with wire net to prevent rodents and other pests entering the fields. Rodents around the experimental site were prevented by mixing rodenticide with groundnut seed which was spread outside the marked out site by using broadcasting method. The treatment plots on each replicates were measured out with the aid of metre rule and pegs were used to demarcate them.
Planting materials and field establishment
The mature groundnut seeds of 2 cultivars (Samnut 23 and Campala) were obtained from the seed production unit, Institute for Agricultural Research Samaru, Ahmadu Bello University, Zaria. The groundnut seeds were sown in 2 different days at interval of 14 days; the first planting date which is June 2, 2012 and the second is June 16, 2012. Three seeds were sown per hole at 25cm x 50cm intra and inter row spacing respectively.
Cultural practices
The groundnut seeds were sown on smooth, uniform, well prepared flat land with good textured soil that has been loosed with the use of hoe to prevent damage to the fragile seeds. Thinning was done after 4 weeks of planting by uprooting one stand from each of the three seedlings that grew from a planting hole on each treatment plot for the growth yield analysis.
Data collection
Planting Samples were collected 4 weeks after planting from the net plot of each treatment plot (Figure 2). The first growth yield analysis was done by uprooting the destructive sample predetermined. The following growth parameters were recorded and growth yield analysis repeated four times for each of the treatments.
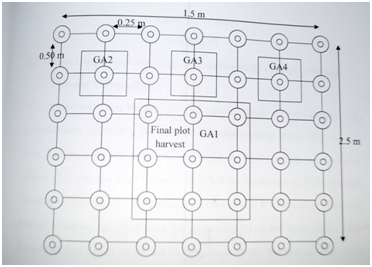
Figure 2 Layout of the treatment plot showing the 42 plants stands used for growth analysis.
Number of pods per plot were counted Data collected were subjected to analysis of variance (ANOVA) and mean separated using Duncan multiple Range Test (DMRT) at 5% degree of precision.
NOTE: GA means Growth Analysi, Represent plant stands
Parameters |
Method used |
First planting date |
June 2, 2012 |
Second planting date |
16-Jun-12 |
Planting depth |
It was measured by using a MR (5cm) |
Days to 50% emergence |
The number of days it took half of the total expected plant stands for each treatment to emerge was recorded |
Days to 50% flowering |
The number of days for half of the total emerged seedlings in each treatment plot to flower |
Number of leaves |
Were counted |
Number of nodules |
Were counted |
Total number of leaves on main stem |
Were counted |
Leaf length |
It was measured by using a metre rule |
Leaf breadth |
It was measured by using a metre rule |
Fresh weight |
The stems, leaves, roots and nodules were bagged in different envelopes, labeled, over dried at 60oC for 72 hours and weighed using sensitive scale |
Date of fertilizer application |
Was recorded |
Fertilizer rate |
0KgN/ha, 5KgN/ha, 10KgN/ha, 15KgN/ha and 20KgN/ha were randomized for the 20 treatment plots on each replicates |
Length of main stem |
It was measured from the planting tip to the root soil level using a metre rule |
Days to 50% pegging |
The number of days it takes half of the total plant stands on a treatment plot to start to forming pods |
Weather parameters |
Collected from LAUTECH weather station; the rainfall, solar radiation, Mm and max temperature, relative humidity were all collected and recorded. |
Date of harvest |
The two varieties, Samnut 23 and Campala were harvested according to their maturity stage from the day of planting |
Number of pods per plant |
Were counted |
Seed weight per plot |
The seeds obtained from each plot were weighed using weighing scale |
Stem length (cm) of groundnut as influenced by cultivars, planting dates and nitrogen rates
The average stem lengths of the groundnuts (Samnut 23 and Campala) were not significantly different at the various weeks after planting (WAP) (Figure 2), (Figure 3). At 2 WAP, Samnut 23 had the higher value of 14.13cm while Campala has the lowest value of 13.27cm. At 6 WAP Samnut 23 also had the higher value of 12.90cm while Campala had the value of 11.30cm and it was the same at 8 WAP and 10 WAP with Samnut23 having the taller plant (23.64cm and 25.66cm respectively) while Campala cultivar had 21.42cm and 19.93cm respectively. The average stem length of the groundnut planted on the planting date 1 (02/06/2012) was not significantly different from that planted on the planting date 2 (16/06/2012) at various weeks after planting. The groundnut planted on the planting date 1 had the higher value of 14.71cm compared to the one planted on the planting date 2, 12.69cm at 4 WAP. The groundnuts planted on the planting date 1 also had the taller plants (13.23, 25.43 and 23.26) at 6 WAP, 8 WAP and 10 WAP respectively. The average stem lengths of groundnut treated with different nitrogen rates were not significantly different. T hose treated with 15kgN/ha had the highest value of 14.97cm while 5kgN/ha treated ones had the least value of 11.42cm at 4 WAP. At 6 WAP, 8 WAP and 10 WAP, the average stem length of groundnut subjected to different nitrogen levels were also not significantly different (Table 1).
Parameter |
Value |
% Nitrogen |
1.13 |
% Potassium |
128.4 |
% Sodium |
83.5 |
% Magnesium |
110.3 |
% Calcium |
380.33 |
% Manganese |
112.6 |
% Zinc |
3.56 |
% Cooper |
2.03 |
% Iron |
108.8 |
% Total Organic Compound |
0.14 |
pH |
6.1 |
% Sand |
76 |
% Silt |
11 |
% Clay |
13 |
Table 1 Physical and Chemical properties of Experimental Site
Number of leaves of groundnut as influenced by cultivar, planting date and N-rates
The average number of leaves of both Samnut 23 and Campala cultivars were not significantly different at 4 WAP and 8 WAP (Table 2). Samnut 23 had 23.47 and 47.80 respectively while Campala had the higher average values of 25.17 and 59.10 respectively. At 6 WAP and 10 WAP, the average numbers of leaves were significantly different with Campala having the higher values of 28.53 and 50.53 respectively. Planting date exerted significantly influence on the number of leaves of groundnut at 4 WAP and 8 WAP. Groundnuts planted on the planting date 1 had the higher value of 27.73 while the one planted on the planting date 2 had higher values of 34.30 and 74.50 at 8 WAP that are not significantly different from those planted on planting date 1 (33.53 and 69.83) at 6 WAP and 10 WAP respectively. The average number of leaves at 4 WAP, 6 WAP and 10 WAP were not significantly different at various levels of nitrogen rates, the groundnut treated with 10kgN/ha had the highest values of 27.17, 39.88 and 81.83 respectively while at the 8 WAP, the ones treated with 10kgN/ha and 15kgN/ha had similar influence with the higher value of 59.58 which were not significantly different with the last average values of 45.00 and 46.33 respesctively.
Weeks After Planting |
4 |
6 |
8 |
10 |
0kgN/ha |
23.42a |
31.50a |
45.00a |
62.83a |
5kgN/ha |
22.08a |
32.50a |
53.33ba |
71.08a |
10kgN/ha |
27.17a |
39.83a |
59.58a |
81.83a |
15kgN/ha |
24.58a |
36.25a |
59.58a |
69.33a |
20kgN/ha |
27.17a |
39.83a |
59.58b |
75.75b |
Cultivars |
||||
Samnut 23 |
23.47a |
28.53b |
47.80a |
50.53b |
Campala |
25.17a |
39.30a |
59.10a |
93.80a |
Planting Dates |
||||
Planting date 1 |
27.73a |
33.53a |
47.50b |
69.83a |
Planting date 2 |
20.90b |
34.30a |
59.40a |
74.50a |
Table 2 Average Number of Leaves of Groundnut as Influenced by Cultivar, Planting Date and N-rates
Means within the interaction table followed by the same letter(s) are not significantly different at 5% probability level according to Duncan Multiple Range Test (DMRT).
Average number of nodules groundnut as influenced by cultivar, planting date and N-rates
At 6 WAP, the average number of nodules counted for Samnut 23 (23.47) was significantly different from Campala which had 25.70 (Table 3). At 4 WAP, 8 WAP and 10 WAP, there was no significant difference in the number of nodules counted. The average numbers of nodules counted on the groundnuts planted on the planting date 2 were not significantly different from those planted on planting date 1 at 6 WAP and 8 WAP at 4 WAP. The groundnut planted on planting date 1 had the highest value of 20.60 which was significantly from the one planted on planting date 2 that had the lower value of 13.90 and at 8 WAP, the value of groundnut planted on planting date 2 differs significantly from the one planted on planting date 1. At 4 WAP, 6 WAP and 10 WAP, the average number of nodules counted at various levels of nitrogen application differ significantly. The groundnut treated with 10kgN/ha had the highest values (19.83 and 28.50) at 4 WAP and 6 WAP respectively while the one treated with 0kgN/ha had the highest value of 68.50 at 10 WAP. At 8 WAP, the groundnut treated with 10kgN/ha had the higher average number of nodules that was 57.00 compared to 31.25, 34.67, 36.42 and 37.67 of 0kgN/ha, 5 kgN/ha, 15 kgN/ha and 20 kgN/ha respectively which were not significantly different from one another.
Weeks After Planting |
||||
4 |
6 |
8 |
10 |
|
0kgN/ha |
14.25a |
15.75a |
31.25b |
68.50a |
5kgN/ha |
17.08a |
26.00a |
34.67b |
37.4a |
10kgN/ha |
19.83a |
28.50a |
57.25a |
56.67a |
15kgN/ha |
18.08a |
26.42a |
36.42b |
53.42a |
20kgN/ha |
17.00a |
12.25a |
37.67b |
43.58a |
Cultivars |
||||
Samnut 23 |
15.53a |
17.87b |
36.73a |
38.37a |
Campala |
18.97a |
25.70a |
42.17a |
65.47a |
Planting Dates |
||||
Planting date 1 |
20.60a |
20.13a |
31.90b |
52.47a |
Planting date 2 |
13.90b |
23.43a |
47.00a |
51.37a |
Table 3 Average Number of Nodules of Groundnut as Influenced by Cultivar, Planting Date and N-rates
Means within the interaction table followed by the same letter(s) are not significantly different at 5% probability level according to Duncan Multiple Range Test (DMRT).
Average number of groundnut pods as influenced by cultivar, planting date and N-rates
The average number of pods of Samnut 23 at 8 WAP was significantly higher than that of Campala which was 5.75 while at 10 WAP, the value for Samnut 23 was not significantly different from that of Campala (Table 4). At 8 WAP and 10 WAP, the average number of pods of groundnut planted on the planting date 1 differs significantly from those planted on the planting date 2. The one planted on the planting date 2 which was lesser value of 14.10 that was not significantly at 10 WAP while at 8 WAP, the groundnut planted on the planting date 2 had the higher value of 9.07. At 8 WAP, the average numbers of pods counted at various levels of nitrogen rate were not significantly different from one another. The groundnut treated with 0kgN/ha had the highest value of 9.42 while the one treated with 20kgN/ha had the least value of 7.25. At 10 WAP, the groundnut treated with 0kgN/ha and 20kgN/ha had the higher value of 15.17 and 13.83 respectively which differ significantly from one treated with 0kgN/ha which had the highest value of 20.92 while the ones treated with 5kgN/ha and 15kgN/ha had the lease values of 10.75 and 10.83 respectively.
Nitrogen Rates |
|
4 |
|
6 |
8 |
10 |
0kgN/ha |
- |
- |
9.42a |
15.17ba |
||
5kgN/ha |
- |
- |
8.25a |
10.75b |
||
10kgN/ha |
- |
- |
8.00a |
20.92a |
||
15kgN/ha |
- |
- |
8.17a |
10.83b |
||
20kgN/ha |
- |
- |
7.25a |
13.83ba |
||
Groundnut Cultivars |
||||||
Samnut 23 |
- |
- |
10.70a |
16.50a |
||
Campala |
- |
- |
5.73b |
12.10a |
||
Planting Dates |
||||||
Planting date 1 |
- |
- |
7.37a |
14.50a |
||
Planting date 2 |
|
- |
|
- |
9.07a |
14.10a |
Table 4 Average Number of Groundnut Pods as Influenced by Cultivar, Planting Date and N-rates
Means within the interaction table followed by the same letter(s) are not significantly different at 5% probability level according to Duncan Multiple Range Test (DMRT).
Average fresh biomass (g) of groundnut as influenced by cultivar, planting date and N-rates
At 4 WAP, 8 WAP, 10 WAP and 12 WAP the fresh biomass of Samnut 23 was not significantly different from that of Campala which had the values of 15.61g, 38.46g and 1136.00g respectively while at 6 WAP, Campala also had the higher value of 17.96g but differs significantly from Samnut 23 which had 11.49g (Table 5). The average fresh biomass of groundnut planted on the planting date 1 differs significantly from those planted on the planting date 2 at 8 WAP, 10 WAP and 12 WAP. Those planted on the planting date 2 had the higher value of 41. 48g at 8 WAP and the ones planted on the planting date 1 had the higher value of 55.52g and 1088.33g at 10 WAP and 12 WAP respectively. At 4 WAP and 6 WAP the values of groundnut planted on the planting date 1 and planting date 2 were not significantly different. At 6 WAP and 10 WAP, the groundnut treated with 10kgN/ha had the highest value of 20.62g which differs significantly from those treated with 0kgN/ha and 15kgN/ha that had the 17.94g and 13.94g respectively while the one treated with 20kgN/ha had the least value of 10.75g. At 8 WAP, the groundnut treated with 10kgN/ha also had the highest average fresh biomass of 47.25g while those treated with 5kgN/ha and 20kgN/ha had the least values of 34.32g and 30.53g respectively. At 12 WAP, the groundnut treated with 15kgN/ha had the highest value of 1212.50g while the ones treated with 0kgN/ha and 20kgN/ha had 1043.30g and 978.39g respe ctively and those treated with 5kgN/ha and 10kgN/ha had the least values of 914.20g and 358.30g respectively.
Weeks After Planting |
|
|
|
|
|
|
4 |
6 |
8 |
|
10 |
12 |
|
Nitrogen Rates |
||||||
0kgN/ha |
17.94a |
13.48a |
35.64ba |
914.20b |
1043.30ba |
|
5kgN/ha |
12.68a |
13.52a |
40.62a |
34.32b |
44.86a |
1212.50a |
10kgN/ha |
20.62a |
18.52a |
47.25a |
49.29a |
358.30b |
978.3ba |
15kgN/ha |
13.94a |
15.59a |
38.56ba |
42.64a |
866.70a |
|
20kgN/ha |
10.75b |
12.53a |
30.53b |
32.56a |
136.00a |
|
Groundnut Cultivars |
||||||
Samnut 23 |
14.76a |
11.49b |
36.06a |
34.07a |
||
Campala |
15.61a |
17.96a |
38.46a |
49.92a |
||
Planting Dates |
||||||
Planting date 1 |
15.43A |
12.65A |
33.04B |
55.52A |
1088.33A |
|
Planting date 2 |
14.94A |
16.81A |
41.48A |
28.47B |
914.33B |
|
Table 5 Average Fresh Biomass of Groundnut as Influenced by Cultivar, Planting Date and N-rates
Means within the interaction table followed by the same letter(s) are not significantly different at 5% probability level according to Duncan Multiple Range Test (DMRT).
Average dry biomass (g) of groundnut as influenced by cultivar, planting date and nitrogen rates
The average dry biomass of both Samnut 23 and Campala were not significantly different at various stages of growth analysis (Table 6). Campala had the lesser value (4.27g) at 6 WAP which was not significantly different from others. At 4 WAP and 12 WAP, the groundnut planted on the planting date 1 had the higher values of 3.51 and 47.33g respectively which significantly differ from the ones planted on the planting date 2 that had 1.82g and 303.33 g respectively. At 6 WAP, 8 WAP and 10 WAP, the values of average dry biomass of the groundnut planted on the planting date 1 and planting date 2were not significantly different from each other. The average dry biomass of groundnut treated with various rates of nitrogen had similar influence, the ones treated with 5kgN/ha had the highest value 3.93g. At 6 WAP, the groundnut treated with 0kgN/ha had the highest value of 26.80g which was not significantly different from one treated with 5kgN/ha that had the least value of 3.34g. At 8 WAP, the groundnut treated with 5kgN/ha had the highest value of 10.54g which was also not significantly different from the one treated with 0kgN/ha that had the least value of 7.02g. At 10 WAP and 12 WAP, the groundnut treated with 15kgN/ha had the highest value of 17.49g and 459.17g respectively while the ones treated with 20kgN/ha had the least value of 11.46g which was not significant at 10 WAP and one treated with 0kgN/ha had the least value (322.50g) that was also not significant.
Weeks After Planting |
|||||
4 |
6 |
8 |
10 |
12 |
|
Nitrogen Rates |
|||||
0kgN/ha |
2.11a |
26.80a |
7.02a |
12.86a |
322.50a |
5kgN/ha |
3.93a |
3.34a |
10.54a |
13.30a |
334.17b |
10kgN/ha |
2.43a |
4.54a |
10.12a |
17.19a |
457.50a |
15kgN/ha |
2.60a |
3.41a |
8.79a |
17.49a |
459.17a |
20kgN/ha |
2.23a |
3.42a |
7.39a |
11.46a |
373.33a |
Cultivars |
|||||
Samnut 23 |
2.53a |
12.34a |
8.68a |
10.78a |
|
Campala |
2.79a |
4.27a |
8.86 a |
18.14a |
415.67a |
Planting Dates |
|||||
Planting date 1 |
3.51a |
12.96a |
8.79a |
14.86a |
475.33a |
Planting date 2 |
1.82a |
3.64a |
8.76a |
14.06a |
303.33b |
Table 6 Average Dry Biomass of Groundnut as Influenced by Cultivar, Planting Date and N-rates
Means within the interaction table followed by the same letter(s) are not significantly different at 5% probability level according to Duncan Multiple Range Test (DMRT).
Seed weight of groundnut as influences by n-rates, cultivar and planting dates
The average seed weight of Campala cultivar was not significantly different from the Samnut 23 cultivar (Figure 2). Campala had the higher weight (61.69g) while Samnut 23 had 58.77g at 12 WAP. Groundnut planted on the planting date 2 had the insignificant higher seed weight of 65.70 compared to that of planting date 1(54.86g). The averae seed weight of the groundnut treated with 15kgN/ha was insignificantly highest (67.34g) compared to that treated with 0kgN/ha (53.25g).
Samnut 23 had significant values of higher plant height, average number of pods and total seed weight at various weeks after planting (WAP) compared to Campala which had the lowest values for the above measured parameters. This is due to the early maturing and high yielding potential of the Samnut 23 cultivar.13 Also, Samnut 23 which is a small-seeded cultivar has thin shells and needs less fertilizer application unlike Campala which is larger-seeded culrivars that have thick shells and requires more fertilizer input to facilitate normal seed development.14 Considering parameters like average number of leaves, average number of nodules, average fresh weight and average dry weight, Campala had the higher values across the weeks after planting. This is attributed to the ability of Campala cultivar to produce high foliage (USDA, 2011).13 The average value of all the parameters measured at various weeks after planting including the final year for the two planting dates are not significantly different from each other but planting date 1 (02/06/2012) had higher values for discontinuous parameters like days to 50% emergence, days to 50% flowering, days to 50% pegging. This is due to the warm temperature required for germination and establishment of seedling during early periods of June (Putnam et al., 1991). Early sown crops also take advantage of early showers and suffer less risk of rosette and other disease.14-21 The groundnut plants that were treated with 10kgN/ha had the highest value which was not significantly different from others. 10kgN/ha also had the highest yields in the number of pods which was significantly different from other rates as well. This is probably due to the percentage of primary elements (N 3.0%, and 1.5% P and 1.5 % N) present in the fertilizer material (Alesinloye Asejere fertilizer) applied. The application of 10kgN/ha of the described fertilizer (organomineral nitrogen) supplied the optimum N.P.K required in the early stages for plant growth, nodulation and disease resistance.6 In addition, Yadava recommended an application of 10kgN/ha as ammonium sulphate at the sowing for soils with moderate to low nitrogen content and this describes the nature of the soils on the experimental plot which has 1.18% of nitrogen content.

©2019 Fagbemigun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.