Advances in
eISSN: 2373-6402


Research Article Volume 8 Issue 4
Department of Crop protection, College of Plant Science and Crop production, Federal University of Agriculture Abeokuta, Nigeria
Correspondence: Otusanya MO, Department of Crop protection, College of Plant Science and Crop production, Federal University of Agriculture Abeokuta, Nigeria
Received: July 16, 2018 | Published: August 30, 2018
Citation: Otusanya MO. Effect of mineral nutrition on anthracnose disease by colletotrichum gloeosporioides Penz and rot by Botryodiplodia theobromae pat in Dioscorea rotundata variety Efuru. Adv Plants Agric Res. 2018;8(4):364-369. DOI: 10.15406/apar.2018.08.00338
An 8-mineral component fertilizer (Bounty) was investigated on anthracnose disease caused by Colletotrichum gloeosporioides and tuber rot by Botryodiplodia theobromae in Dioscorea rotundata var. Efuru, the most popular white guinea yam variety in South West Nigeria. The field plot design was RCBD with two treatments of 0 and 0.6ml l-1 Bounty applied at 3 MAP (months after planting) and three replicates. Anthracnose incidence at 5 MAP was 25% (both treatments) and reduced to 10%, in both treatments at 6 MAP (with no significant differences). Anthracnose severity score of 1, which is resistant, was scored both at 5 MAP and at 6 MAP. Leaf analysis at 6 MAP indicated an increase of 3 phytoanticipins namely Tannin (4.96), lignin (2240), flavonoid (763.33) in fertilized plants over the control which had 3.59, 2130 and 736.67mg/100 dry matter respectively. Leaf N, P, S and Fe in the fertilized plants with the values 538.67, 63.11, 1086, 0.847mg/100g dm respectively were higher than the control. Leaf N, P, S and Fe had significant correlation with leaf tannin (r=0.9221, 0.9616, 0.9531, 0.9439 respectively). Leaf Fe correlated significantly with leaf P and leaf P with leaf lignin (r=0.9638 and 0.8426 respectively). Anthracnose incidence at 6 MAP correlated negatively with leaf flavonoids (r= 0.8402). Increase of the flavonoid, lignin, and tannin with combined effect of the four minerals enhancing (significant positive correlations) the phytoanticipins is responsible for anthracnose resistant status in the variety. But control plants were also resistant indicating that the leaf calcium of control plants may be optimum (as fertilized plants had significantly lower calcium), in this variety, and additional calcium from the soil amendment was not beneficial. Also calcium level in the leaves of control plants had exhibited enough structural integrity against the anthracnose pathogen. Moisture, ash, crude protein, crude fiber, fat, carbohydrate as well as Ca, Mg, Fe content were not significantly different in the tubers. Tuber N, P, S as well as phenol were higher in the fertilized plants than the control. Mean infection (1.13%) after 2 weeks incubation of tubers with B. theobromae is 40 to 70% lower than is reported for calcium fertilization of Dioscorea species. Weight loss after incubation was 0% in both treatments. Tuber calcium content which was over 10mg/100gdm was not significantly different in both treatments and may be optimum for tubers of variety Efuru, for reduction of infection/weight loss by B. theobromae. Nitrogen, the highest component (15%) of Bounty fertilizer was higher in the leaves of fertilized plants, and correlated significantly with tuber phenol (r=0.9342), confirming role of the minerals in the control of both anthracnose and in the reduction of tuber infection/weight loss in variety Efuru. Yield in this study is 15 to 15.3 tons ha-1, comparable with the 10 to 15 tons ha-1 expected yield under continuous cultivation with 400 kg (8 bags) NPK 15-15-15 per hectare in the forest zone of Nigeria.
Yams, Dioscorea species are a popular staple in the tropical regions of the world. The major constraints to production include the field disease anthracnose caused by Colletotrichum gloeosporioides Penz., which is currently prevalent on all cultivated species.1 Tuber rots by microorganisms especially fungi account for a greater loss in storage than any other single cause (Onwueme, 1978). Tuber losses in Nigeria, the world’s leading producer of yams,2 has made this staple costly in the scarce period of the year and export to be as low as 0.03%. Aspergillus niger, Botryodiplodia theobromae and Rhizopu sspecies are fungi consistently found in association with deteriorating yam tubers.3,4 Mono cropping for smallholder farmers is not an easy task because yams drain heavily on soil nutrients5 and 2 years (minimum) traditional fallow is usually maintained for soil replenishment in Nigeria. However, continuous cultivation or mono cropping is practiced by richer farmers with pesticides usage and fertilizer (NPK) application to maximize yield. Mineral nutrition for management of Dioscorea diseases, both in the field and in storage is desired as it is cost effective and reduces pesticide hazards in the environment. This study investigated the effect of an 8-mineral component fertilizer (Bounty) on anthracnose disease and tuber rot and weight loss by Botryodiplodia theobromae in D. rotundata variety Efuru, on a field plot that had been mono cropped to Dioscorea species in two previous consecutive years with only calcium nitrate fertilization (soil amendment). Leaf and tuber analysis were carried out to determine effect of Bounty fertilizer on mineral levels, phytochemical, phenol and proximate content.
Field plot
The field plot had been mono cropped to Dioscorea species with only calcium nitrate fertilizer application (by soil amendment) for two previous consecutive years (2015, 2016) before this study was carried out in the third year (2017). It was located at the Teaching and Research Farms of the Directorate of University Farms (DUFARMS), Federal University of Agriculture, Abeokuta, Ogun State (South West Region), Nigeria. The site is sandy-loam. The site was cleared manually with hoes and machetes and old stakes were burnt before the marking out, in the first week of April (onset of steady rains). A field area of 14m by 9.5m was used with a clean border of 1 m all round. The design was RCBD (randomized complete block design) with 2 treatments and 3 replicates. The 2 treatments were 0 ml l-1 and 0.6 ml l-1 Bounty fertilizer. Mounds were 1m by 1m, 80cm high with a 2m length bamboo stake each. Inter-row and intra-row spacing was 1m and 0.5 m respectively. There were 54 plants with 18 plants per replicate.
Planting and fertilization
Seed yams of average size 0.5 to 0.6 kg of D. rotundatavar Efuru sourced from farmers plots within Ogun State, were planted, one per mound. Efuru is about the most popular local white guinea yam variety in South West Nigeria. Bounty fertilizer which was applied at 3MAP contains 8 minerals in the following combination: Ca 7%w/w, N 15%w/w, Mg O 0.5%w/w, Zn 2%w/w, Mn 1%w/w, Cu 0.5%w/w, Fe 2%w/w and B 0.0025w/w. It was prepared with distilled de ionized water, at the rate 0ml l-1 (control) and 0.6ml l-1. Grooves were made in each mound, of about 20cm depth and of 25cm radius around the yam root,5 and the fertilizer poured evenly into it, then the groove was covered well with soil. Weeding was done as necessary to disallow weed competition with the crop.
Disease incidence and severity
Anthracnose incidence was assessed by counting plants with disease symptoms in each replicate at 5MAP and also at 6MAP. Percent incidence was calculated using the formula:
Disease severity was assessed using the Anthracnose severity scale in (Figures 1-3).6
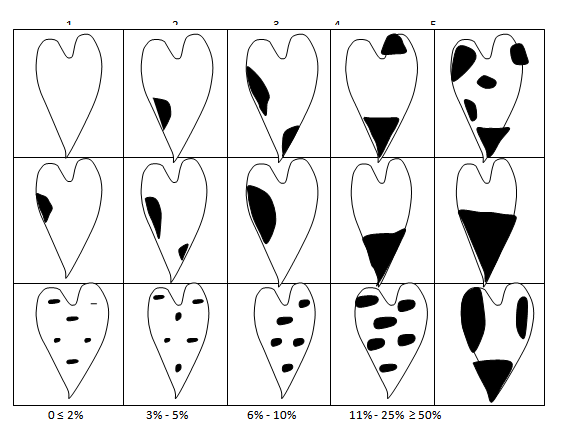
Figure 1 Yam leaf anthracnose disease assessment diagram + lesions on petiole and stem.
Modified from Green (1994)
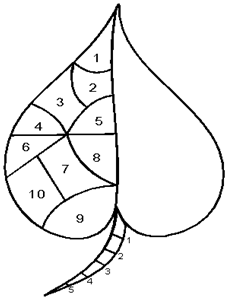
Figure 2 Leaf anthracnose assessment.
Petiole 1/5x100= 20%
Leaf BLADE 1/10= 5%; 2/10=10% ETC.
10 Leaves UPPER PORTION (young=tender/lighter texture)
10 Leaves MIDDLE PORTION (maturing=green/expanded/big)
10 Leaves LOWER PORTION (fully expanded +well-formed Cuticle/texture leathery)
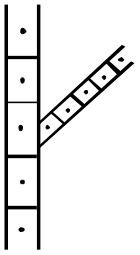
Figure 3 Vine and Petiole anthracnose assessment.
5 Sections
5 Sections -2 vines only TDA
Vine only TDR
Vines only TDA (local)
Estimated % area scored per Vine/Petiole (5 sections)
Leaf sampling and analysis
Sampling was done at 6MAP, along a diagonal transect of the field plot, for randomization of treatments. From each tagged/labeled plant, 12 leaves including the petioles were collected (4 each from top, middle and bottom, just above the mound) in the early hours, (7.00a.m) into new large brown envelopes and transferred to the Crop Protection Laboratory, COLPLANT (College of Plant Science and Crop Production), FUNAAB (Federal University of Agriculture, Abeokuta). Leaves were rinsed in plastic bowls containing de ionized water, allowed to dry in labeled trays under a low-speed ceiling fan and then transferred in the labeled trays to the top of raised wooden, netted yam storage structures (under shade) in the COLPLANT Screen house for further drying for 2 to 3 days. The dried leaves were then milled to powder with a waring 2 speed Saisho blender at the Biotechnology Laboratory, FUNAAB. Analysis was carried out using routine methods of the A.O.A.C. (1990), for tannin, lignin flavonoid and minerals namely Calcium (Ca), Nitrogen (N), Magnesium (Mg), Iron (Fe), Potassium (K), Phosphorus (P) and Sulphur (S), in the Biotechnology Centre and the Biological Sciences Tetfund Laboratory, FUNAAB.
Tuber sampling, infection/weight loss experiment
Tuber sampling at 6MAP, along the same diagonal transect (as for the leaf sampling) for random treatment selection was done, using wooden spoons. Tubers were sampled for the tuber tissue analysis as well as the infection/weight loss experiment. Soil was pushed gently (to avoid bruising tubers) away from the roots to expose the tubers. The number of tubers needed were each turned clockwise until they snapped free of the shoots. Sampled tubers were allowed to dry in the field and transferred to the COLPLANT Screen house. They were cleaned carefully of soil with a soft cloth, labeled and weighed with a mettler balance. Weight of each tuber was measured with a Mettler balance before inoculation. Inoculation of tubers was done in an Inoculation Hood Structure in the Crop Protection Lab., COLPLANT Building, and FUNAAB. Two cork borers (6mm and 4 mm), scalpel and a pair of forceps were surface-sterilized by dipping into 80% ethanol and flaming to red-hot over a lighted spirit flame bottle. They were allowed to cool, slanted against another cork borer. Inoculation point (inoculation site) on each tuber was surface-sterilized with cotton wool dipped in methylated spirit (70%). About 10–12mm deep (depending on size of the tuber) holes were bored into the inoculation site with the sterilized 6mm cork borer. The incised tissue was removed with the scalpel and forceps. One 4 mm disc of a 7-day old pure culture (on potato dextrose agar) of Botryodiplodia theobromae (originally from a partially rotted Efuru tuber) was placed in the hole, with the sterilized 4 mm cork borer. The cutout tissue was then replaced and the incision sealed with vaseline, (petroleum jelly). The inoculated tubers were arranged in the yam storage structures in the COLPLANT Screen house and incubated for a period of two weeks. At the end of the 2 weeks, each tuber was cleared of vaseline with spatula and cotton wool. Weight of each tuber was again recorded. Each tuber was then cut through the inoculation site, into two halves with a steel knife. Infected tissue was cut out from each half with a scalpel on to a pre-weighed sheet of aluminum foil, and weights of infected tissue determined with an electronic balance.
Percent weight loss was calculated with the formula;
Where A = Weight of tuber at the beginning of the experiment and B= Weight of tuber at the end of the experiment
Percent infection was calculated with the formula:
, and A is weight of tuber at the beginning of the experiment.
Where X = Weight of infected tissue and Y = Percentage weight loss
Proximate content, phenol and mineral content analysis of tubers
A second set of the sampled tubers were used for tuber tissue analysis. Triplicate tubers were used per treatment. Dry matter, moisture, ash, fat, crude protein, crude fiber and carbohydrate content were analyzed according to routine methods of the A.O.A.C.,7 in the Biological Sciences Tetfund Laboratory and Biotechnology Centre, FUNAAB. Phenol content and minerals namely Nitrogen, Calcium, Magnesium, Phosphorus, Potassium, Iron and Sulphur were analyzed also according to the methods of the A.O.A.C.7 in the two laboratories, mentioned above.
Yield at 6MAP
Yield assessment was done 24 hours (1 day) after tuber sampling for tissue analysis. Tubers were harvested by moving/carefully pushing soil away from plant roots so as not to bruise tubers, using smooth wooden spoons and turning those clock wise until they snapped free from the shoot. Tubers were left to dry on the mounds for about 3 hours before counting numbers per plant and determining weight of tubers per plant with a top loading field balance.
Data analysis
Data obtained were subjected to analysis of variance using SAS 2000, version 9.1.3. Means were separated using Tukey’s (HSD) test.
Anthracnose disease incidence and severity
Table 1 gives anthracnose disease incidence at 5MAP as 25% in both the control and the Bounty-fertilized plants with no significant difference between them. Four weeks after at 6MAP, incidence had reduced in both treatments. It was 12.5% in the control plants and was significantly lower in the Bounty-fertilized plants where it was 8.33%. Even though anthracnose incidence was observed as recorded, severity was low. Severity score of 1 which is the category of resistant was recorded at 5MAP and also at 6MAP (Table 2).
Bounty fertilizer treatment (ml l-1) |
Disease incidence (%) at 5MAP |
Disease incidence (%) at 6MAP |
||
0 |
25.00a |
12.50a |
||
0.6 |
25.00a |
8.33b |
|
|
Table 1 Anthracnose disease incidence in Bounty-fertilized Dioscorearotundata variety EFURU at 5MAP and 6MAP
Means in a column followed by the same letter are not significantly different at P ≤ 0.05 (Tukey’s HSD test)
Bounty fertilizer treatment (ml l-1) |
Disease severity score at 5MAP |
Disease severity score at 6MAP |
|
0 |
1 |
1 |
|
0.6 |
1 |
1 |
|
Table 2 Anthracnose disease severity score in Bounty-fertilized Dioscorearotundata variety EFURU at 5MAP and 6MAP
Means in a column followed by the same letter are not significantly different at P = 0.05 (Tukey’s HSD test)
Phytochemical (flavonoid, tannin and lignin) and leaf minerals
Flavonoid content in the Bounty-fertilized plants was 763.33mg/100gdm significantly higher than in the control which was 736.67mg/100gdm (Table 3) Tannin and Lignin were also significantly higher in the Bounty-fertilized plants, that is 4.96 and 2240 mg/100gdm respectively than the control which were 3.59 and 2130 mg/100gdm respectively. Calcium and Magnesium were lower in the fertilized plants, that is 883.67 and 198.07 mg/100gdm respectively than the controls which were 1045.33 and 203.17mg/100gdm respectively. Leaf phosphorus, nitrogen, sulfur and iron were all significantly higher in the Bounty-fertilized plants, that is 63.11, 538.67, 1086 and 0.847mg/100gdm respectively than in the control which were 60.24, 516.67, 966 and 0.816 mg/100gdm as shown in Table 2. Only leaf potassium was comparable/similar in both treatments. The values are 66.07mg/100gdm in the control and 63.99mg/100gdm in the fertilized plants (Table 3).
Bounty fertilizer treatment (ml l-1) |
Phytochemical |
|
|
Minerals |
|
|
|
|
|
|
Lignin |
Flavonoid |
Tannin |
Ca |
Mg |
K |
P |
N |
S |
Fe |
|
0 |
2130.00b |
736.67b |
3.57b |
1045.33a |
203.17a |
66.07a |
60.24b |
516.67b |
966.00b |
0.816b |
0.6 |
2240.00a |
763.33a |
4.96a |
883.67b |
198.07b |
63.99a |
63.11a |
538.67a |
1086.00a |
0.847a |
Table 3 Phytochemical composition (mg/100gdm) and minerals (mg/100gdm) in leaves of Dioscorearotundata var. EFURU at 6 MAAP
Means in a column followed by the same letter are not significantly different at P ≤ 0.05 (Tukey’s HSD test)
Correlation of anthracnose incidence or severity, leaf flavonoid, tannin, lignin and leaf minerals
Only significant correlations (P=0.01 or 0.05) are presented in Table 4. Leaf nitrogen correlated significantly with leaf tannin (r=0.9221) and leaf tannin correlated significantly with leaf lignin (r=9090) as shown in Table 4. Leaf lignin also correlated significantly with leaf flavonoid (r=0.8848). Nitrogen in the leaf correlated with leaf phosphorus (r=0.9857), and leaf phosphorus with leaf lignin (r=0.8426) (Table 4). Iron correlated with tannin, nitrogen and phosphorus with the values r=0.9439, r=0.9773 and r=0.9638 respectively, (Table 4). Phosphorus correlated with tannin, r=0.9616. Iron correlated with sulfur (r=9904) and sulfur with tannin (r= 0.9531). Nitrogen also correlated with leaf sulfur (r=0.9769) and leaf sulfur with phosphorus (r=0.9776). There was also a significant negative correlation between leaf flavonoid and disease incidence at 6MAP with the value of r = -0.8402.
|
K |
Lignin |
Tannin |
Fe |
Calcium |
N |
P |
Flavonoid |
Flavonoid |
0.8848 |
|||||||
Lignin |
0.909 |
0.8426 |
||||||
Tannin |
0.9439 |
0.9221 |
0.9616 |
|||||
Fe |
0.9773 |
0.9638 |
||||||
S |
0.9531 |
0.9904 |
0.9769 |
0.9776 |
||||
DI 6 MAP |
-0.8402 |
|||||||
P |
0.9857 |
|||||||
Phenol |
|
|
|
|
|
0.9342 |
|
|
Table 4 Significant Correlations of Anthracnose Incidence/leaf minerals/Anticipins/tuber phenol in D. rotundata var. EFURU
Correlations significant at P=0.01, 0.05
Infection and weight loss in tubers inoculated and incubated with B. theobromae for 2 weeks
Infection was not significantly different in the two treatments, 1.29% (control) and 1.32% (0.60ml l-1 treatment). There was no weight loss, either in the control or the Bounty-fertilized tubers in the two weeks as shown in Table 5.
Bounty fertilizer treatment (ml l-1) |
Infection (%) |
Weight loss (%) |
0 |
1.29a |
0 |
0.6 |
1.32a |
0 |
Table 5 Infection and weight loss in tubers of Bounty-fertilized Dioscorearotundata variety EFURU after 2 weeks incubation with Botryodiplodiatheobromae
Means in a column followed by the same letter are not significantly different at P ≤ 0.05 (Tukey’s HSD test)
Proximate content and phenol content of tubers at 6MAP
Moisture content was over 10% in the control and Bounty-fertilized tubers with no significant differences between them. None of the proximate contents were significantly different from one another in the two treatments as shown in Table 6. Crude fiber was over 11% dm in both treatments. Carbohydrate was about 80% in both treatments. Ash and crude protein were over 2% and 5% respectively in both treatments (Table 6). Fat content was low, and less than 1% in both treatments as shown in (Table 6). Phenol, however, was significantly higher in Bounty-fertilized tubers 123.34mg/100gdm than the control which was 116.17mg/100gdm. Tuber phenol content correlated significantly with value r= 0.9342 with leaf nitrogen (See Table 4). Calcium a mineral is put in the column next to phenol in the Table so as to compare their relative values. Tuber calcium was about 8% and 10% of phenol content in the control and fertilized tubers respectively.
Bounty fertilizer treatment (ml l-1) |
Phenol |
Calcium |
Dry matter |
Moisture |
Ash |
Crude fibre |
Crude protein |
CHO |
Fat |
0 |
116.17b |
11.38a |
88.76a |
11.24a |
2.54a |
11.01a |
5.87a |
80.05a |
0.53a |
0.6 |
123.34a |
10.23a |
88.81a |
11.19a |
2.55a |
11.20a |
5.96a |
79.77a |
0.53a |
Table 6 Proximate composition (%dm), calcium and phenol (mg/100gdm) of tubers of Dioscorearotundata variety EFURU at 6 months after planting
Means in a column followed by the same letter are not significantly different at P ≤ 0.05 (Tukey’s HSD test)
Mineral content of tubers
Iron, magnesium, and calcium in the two treatments were not significantly different from one another as shown in Table 7. Nitrogen, phosphorus and sulfur were significantly higher in the Bounty-fertilized tubers than in the control. Tuber nitrogen was relatively higher than the other minerals at over 800mg/100gdm. Sulfur was also high at over 750mg/100gdm. Tubers of the control plot however had significantly higher potassium (84.40 mg/100gdm) than the fertilized tubers (79.75mg/100gdm).
Bounty fertilizer treatment (ml l-1) |
Phenol |
Ca |
Mg |
K |
N |
P |
S |
Fe |
0 |
116.17b |
11.38a |
25.09a |
84.40a |
801.33b |
64.09b |
752.00b |
0.82a |
0.6 |
123.34a |
10.23a |
24.69a |
79.75b |
848.33a |
68.29a |
784.33a |
0.96a |
Table 7 Mineral content (mg/100gdm) and phenol (mg/100gdm) in tubers of Dioscorearotundata variety EFURU at 6 months after planting
Means in a column followed by the same letter are not significantly different at P ≤ 0.05 (Tukey’s HSD test)
Yield at 6MAP
Tuber number per plant was 1.56 and 1.78 in the control and fertilized plants respectively, with no significant differences between them (Table 8). Tuber weight per plant was 1.53kg in the control and 1.50kg in the Bounty-fertilized plants. The equivalent of these two in tons per hectare is 15.30 tons ha-1 and 15 tons ha-1.
The field plot that was used in this study had been mono cropped to Dioscorea species with only calcium nitrate fertilizer application (soil amendment) in the two previous and consecutive years (2015 and 2016). This study was carried out in the same field plot in the 3rd year (2017) with the application of Bounty fertilizer. Bounty is a fertilizer composed of eight (8) minerals in the following combinations: Calcium 7%w/w, Nitrogen 15%w/w, Magnesium oxide 0.5%w/w, Manganese 1%w/w, Zinc 2%w/w, Iron 2%w/w and Boron 0.0025%w/w. The objective of this study was to determine the effect of Bounty fertilizer containing 8 minerals on anthracnose disease as well as tuber rot by B. theobromae on D. rotundata var. Efuru on a plot that had been mono cropped to Dioscorea species with Ca(NO3)2 application by soil amendments in two previous consecutive years. This is because calcium carbonate by soil amendment had reduced infection and weight loss by Aspergillus niger and Botrypodiplodia theobromae in two improved varieties of Dioscorea rotundata and Dioscorea alata, in a previous report 8 and two Ca (NO3)2 foliar sprays within the 9 month growth cycle, had limited anthracnose to a tolerant level and increased yield in 3 varieties of Dioscorea rotundata and Dioscorea alata.6 Thus the plants in this study were observed closely from planting. Fertilizer (Bounty) was applied by soil amendment at the recommended period of fertilizer application in Dioscorea species of 3 months after planting.5Anthracnose disease incidence was 25% at 5MAP. However, at 6MAP, disease incidence had reduced to a mean of over 10% in both treatments in the field. Anthracnose spots had also become fewer and smaller at the maximum leaf production stage that is 12 weeks after planting to 22 weeks after planting.9 Severity score of 1 which is the category of resistant was recorded on all plants at 5MAP as well as at 6MAP. Bounty fertilizer increased all three phytochemical or phytoanticipins namely flavonoid, tannin and lignin in the leaves of the fertilized plants compared to the control. There was also an increase of Nitrogen, Phosphorus, Sulphur, and Iron in leaves of the fertilized plants over the control. Leaf Calcium, Magnesium and Potassium, however, did not increase in the fertilized plants.
Three of the minerals that had been increased by fertilizer application namely N, P and S correlated positively and significantly with the leaf phytoanticipin tannin. Leaf Iron also correlated positively with leaf tannin and leaf phosphorus, and leaf phosphorus in turn correlated positively with leaf lignin. Leaf iron correlated positively also with leaf sulphur. Leaf lignin correlated positively with leaf flavonoid content and leaf tannin content. Furthermore, disease incidence at 6MAP correlated negatively with leaf flavonoids. It may be said therefore that Bounty fertilizer increased the three phytoanticipins in the leaves to exhibit their various antimicrobial effects on the anthracnose pathogen. Bounty fertilizer also increased four leaf minerals (N, P, S, Fe) which in turn promoted or enhanced (in terms of positive correlation) further the level of activity of the three phytoanticipins in the leaves, resulting in the resistance status (severity score 1) in the variety to Anthracnose disease. Leaf calcium in the control plants was higher than in the Bounty-fertilized plants. This may be probably because one or more minerals in the Bounty-fertilized plot prevented uptake of more calcium there (this needs future investigation). However severity score of 1 that is resistant occurred in both treatment plots. Calcium maintains structural integrity in plant cell walls by formation of calcium pectate (Marshner, 1995).Thus calcium uptake may have been adequate in the leaves of control plants to exhibit enough structural integrity against the anthracnose pathogen. All proximate components were similar in fertilized and control tubers. However, Bounty fertilizer increased phenol in the tubers of the fertilized plants. Phenol has been implicated in resistance to disease in crop plants.10 Tuber Nitrogen, Phosphorus, Sulfur, and iron were also higher in the fertilized plants, only potassium was lower compared to the control. Calcium uptake/ content in the tubers were not significantly different in fertilized plants and control plants. As calcium had been applied in the field for the third year running, it may be said that D. rotundata variety Efuru tubers may have taken up the optimum calcium content.
Weight loss was nil (0%) in both treatments after incubation of tubers with B. theobromae for two weeks. Also mean infection in the two treatments (as they were not significantly different from one another) was 1.31%, which is 40 to 70% of infection by B. theobromine in an improved variety of white guinea yam (D. rotundata TDr 131) after calcium fertilizer application.8 Phenol content of tubers of the fertilized plants was higher than that of tubers of the control treatment. Despite the higher phenol content of fertilized plant tubers infection of both treatments were not significantly different and were both low, coupled with no weight loss after 2 weeks infection. Suffice again to say that tuber calcium content in both control and fertilized plant tubers may be optimum and structural integrity had been conferred to the tuber cells, resulting in the comparable low infection and 0 % weight loss. Leaf nitrogen correlated positively with tuber phenol in this study (r=0.9342). Thus nitrogen which is the highest component in Bounty fertilizer enhanced leaf nitrogen as well as tuber phenol, confirming the role of the minerals in Bounty fertilizer in the control of anthracnose disease and the reduction of tuber weight loss and tuber infection by Botryodiplodia theobromae in Dioscorea rotundata var. Efuru. This white guinea yam variety (Efuru) is more or less the most popular in South West Nigeria, and it may be recommended that Bounty fertilizer be used in its production especially where yam mono cropping is desired. Yield turn out at 6MAP in this study was 15tons ha-1 to 15.3tons ha-1, comparable to the 10 to 15tons ha-1 expected yield with good management, under continuous cultivation of 400kg (8bags) of NPK 15-15-15/ha (60kg N, 60kg P2O5 and 60 kg K2O/ha) in the forest zone of Nigeria (ICS–Nigeria).11
None.
The author declares there is no conflict of interest.

©2018 Otusanya. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.