Advances in
eISSN: 2373-6402


Research Article Volume 1 Issue 4
Department of Biotechnology, Nigeria Institute for Oceanography and Marine Research, Nigeria
Correspondence: Ayo Olalusi CI, Department of Biotechnology, Nigeria Institute for Oceanography and Marine Research, P.M.B. 12729, Victoria Island Lagos, Nigeria, Tel +2348051975562
Received: August 27, 2014 | Published: October 20, 2014
Citation: Ayo OCI, Mojekwu T, Adeleke TA, et al. Digestive enzymes assay and haematological profile of clarias gariepinus juveniles fed with probiotics supplemented diets. Adv Plants Agric Res. 2014;1(4):158-162. DOI: 10.15406/apar.2014.01.00025
This study was carried out to access the effect of feeding probiotics on haematological parameters of Clarias gariepinus juveniles as bio indicator of health status and to determine the enzymatic activities and the efficiency of Clarias gariepinus to use feeding components. The fish were fed for 70days with 7 different diets supplemented with 10ml bacteria suspension kg-1 diet of 3.2X108 CFUg-1 probiont concentration: Lactobacillus brevis1 (PRO1), Lactobacillus plantarum (PRO2), Pediococcus pentosaceus (PRO3), Lactobacillus brevis1, + Lactobacillus plantarum (PRO4), Lactobacillus brevis1 + Pediococcus pentosaceus (PRO5) Lactobacillus plantarum + Pediococcus pentosaceus (PRO6) with the experimental feed. The control diet was without probiotics (PRO7). Haematological profile and digestive enzymes assay of Clarias gariepinus fed with probiotics were evaluated after 30, 50 and 70days of feeding trial. At the 30, 50 and 70 of feeding experiment, two fish from each replicate were taken and blood was collected from caudal vein using sterile syringe, which was previously rinsed with 2.7% EDTA solution as an anticoagulant. Two fish specimens were also taken from each replicate for the enzymatic assays, sacrificed and gut removed to study the enzymatic distribution. Packed cell volume (PCV) and white blood cell (WBC) were determined using haematocrit reader and improved counting chamber respectively while the digestive enzymes assay was also determined using Fortress diagnostics kits. Results showed that there was an increase in blood parameters of Clarias gariepinus fed probiotics diet treatment after 30, 50 and 70 days compared to the control diet. Highest mean lipase and amylase values of 0.2824±0.11 and 0.0657±0.02, 0.2912±0.09 and 0.0651±0.01, 0.3421±0.13 and 0.0837±0.012 were recorded in the gut of fish fed with PRO2 (Lactobacillus plantarum) after 30, 50 and 70 days of feeding experiment respectively. Fish fed with L. plantarum (PRO3) recorded highest WBC value and least PCV value compared to other treatments but no significant difference (P>0.05) in all the treatments. The increase in WBC counts recorded in this research when compared with other treatments could be due to the attempt of the fish to fight against pathogens which augmented the production of more WBC to improve the health status of the fish.
Keywords: digestive enzymes, haematological profile, clarias gariepinus, probiotics
EDTA, ethylene di amine tera acetic acid; PCV, packed cell volume; WBC, white blood cell; MRS, de man rogosa and sharpe; API, analytical profile index; EPS, ethylidene protected substrate; ANOVA, analysis of variance
Probiotics are viable cell preparations that have beneficial effects on the health of a host by improving its intestinal balance via improved feed value, enzymatic contribution to digestion, inhibition of pathogenic microorganism, anti-mutagenic and ant- carcinogenic activities, growth promoting factors and an increased immune response.1 Probiotics are studied separately as single- dose bacteria incorporated into fish feed for growth studies but recently, fewer studies have been conducted on the use of two or more bacteria as a double- dose of probiotic mixture in fish feed.2–4 Buddington et al.,5 reported that the determination of digestive enzymes activities may provide information about the whole digestive capacity and the efficiency of species reared to use feeding components. Knowledge of the digestive capability of reared species is necessary for adapting dietary formulation to the functionality of the digestive tract and represents one of the most important objectives of research in fish nutrition.6 As digestive enzymes can be used as surrogate parameters to predict the ability of fish to use different nutrients, in vitro measurements of digestive enzymatic activity may allow adapting artificial feeding to the nutritional needs of fish.7 Endogenous digestive enzymes in fish have been studied by several scientists.7–9 Haematologic evaluation can be useful in assessing the health status of fish and monitoring stress responses. However, haematological parameters are closely related to the response of the animal to the environment, an indication that the environment where fishes live could exert some influence on the haematological characteristics.10 The primary objective of this project was to evaluate the effect of different dietary probiotics groups on the activity of digestive enzymes and haematological status of C. gariepinus fed with probiotics (Lactobacillus brevis1, Lactobacillus plantarum and Pediococcus pentosaceus) supplements.
Experimental fish and design
Clarias gariepinus (9.02±0.021g) were obtained from a commercial fish farm in Ogun state, Nigeria and transported alive in a plastic kegs containing aerated water. They were acclimatized for a period of two weeks in fiber tanks (90mx30mx20m) filled with water and continuous aeration was provided using electric air pumping compressors. Fish were fed with 40% crude protein commercial feed prior to commencement of the experiment. Forty (40) fishes (11.90g mean weight) were randomly distributed into (20mx30mx40m) 21 plastic tanks each in triplicates and fed thrice daily at 3% of their body weight throughout the period of the experiment for maximum utilization of the feed and to prevent wastage. All the groups, except or the control were fed with feed incorporated with probiotics (3.2x108CFUgˉ¹). Water quality parameters were monitored throughout the feeding trial which could also be contributing factors in boasting the condition of the fish apart from the feed. The mean temperature, pH, alkalinity, hardness and dissolved oxygen of the water used were 27.4±0.01°C, 6.51±0.02, 193.3±5.21mgl-1 (as HCO3 -), and 227.5±22.1mg l-1 as CaCO3 and 6.56±mgO2 l-1 respectively. The water was changed thrice daily. Four fish were taken per tank for the analysis, weight taken with a weighing machine (OHAUS model 5000g). The blood was taken and the gut removed for the haematological analysis and digestive enzyme assay respectively.
Culture of probiotics bacteria for incorporation
Bacteria strains (Lactobacillus brevis1, Lactobacillus plantarum and Pediococcus pentosaceus) were cultured and isolated from the gut of some culturable fish species from marine, freshwater and brackish water respectively using modified MRS (de Man Rogosa and Sharpe) broth and MRS agar. The 0.05% cysteine was added to MRS to improve the specificity of this medium for isolation of lactobacillus. 1ml of each sample was dissolved in 9ml of MRS broth at pH 6.2, shaken homogeneously and incubated at 37°C anaerobically for 24hrs. The cultures were subjected to five subculture at 37°C under low pH (pH 4.5) and anaerobic condition in the presence of 10% CO to remove unwanted bacteria. After incubation, the cells were harvested by centrifugation (2500 rpm for 30minutes) washed with phosphate- buffer saline (PBS, pH 7.0) and re-suspended in PBS. Biochemical tests were carried out (Gram staining, catalase, endospore and motility test)
And identification was based on the characteristics of the lactobacilli as described in Bergey’s Manual of Determinative Bacteriology (Azcarate-Peril), fermentation of different carbon sources (API 50 CHL, BioMerieux) on the API 50 CH strip to be studied. They were subsequently preserved in 20% glycerol at -80 ºC and kept as culture for further use.
Preparation of probiotics diets
The probiotic diets were prepared by gently spraying the required amount of bacterial suspension on the control diet. McFarland standard of 1.0 with cell count density of 3.2 x 108 cells were employed for quality control. The turbidity of the bacterial suspension was adjusted. Mixing of the feed was done in an improvised bowl to obtain a final probiont concentration. Seven different experimental diets with or without probiotics supplementation namely PRO1 (L. brevis1), PRO2 (L. plantarum), PRO3 (P. pentosaceus2), PRO4 (L. brevis1 and L. plantarum), PRO5 (L. brevis1 and P. pentosaceus2), PRO6 (L. plantarum and P. pentosaceus) and PRO7 (experimental diet without probiotics - control) were formulated.
Digestive enzymes assays
Tissue homogenates of intestine were prepared with chilled sucrose solution (0.25m) and the different digestive enzymes were analyzed using standard procedures (fortress diagnostic kits) as described by Fortress diagnostic limited , Northern Ireland, U.K. Lipase method was based on cleavage of a specific chromogeric lipase substrate 1,2 O-diauryl-rac-glycerol-3- glutamic acid (6-methylresorufin) ester emulsified with bile acids. The combination of bile acid and calipase was used in this assay to detect the lipase activity. In this assay the rate of formation of methylresorufin was measured photometrically and is directly proportional to the activity of lipase.
Amylase activity using kinetic method was based on the well proven cleavage of 4,6-ethylidene- (G7)-1,4-nitrophenyl (G1)-D-maltoheptaose (ethylidene protected substrate- EPS) by amylase and subsequent hydrolysis of all the degradation products to P- nitrophenol with the aid of glycosidase (100% chromophone liberation). The color intensity of the P- nitrophenol formed was directly proportional to the α-amylase activity and was measured photometrically. Enzyme activities were calculated as: μmol/ml x final vol. in well x dilution factor/ sample volume x incubation time.
Haematology determination
Blood was collected directly from the tail fin into EDTA capillary tube, about ¾ full. This was then sealed at one end with a sealant. The sample was then put in a haematocrit centrifuge and spun for 5mins at 12,000 rpm. The value of the PCV was then read off using a haematocrit reader and recorded. For white blood cell (WBC), blood was collected directly from the tail fin (1part) into a tube containing 19 parts of Turk’s solution. This was gently mixed. The improved Neubeur counting chamber was assembled and charged with the prepared sample and examined microscopically. White cells in each of the four outer cells of the chamber were counted and the value calculated from the formula:
N×DF×106A×D per liter
Where N=number of cells counted, DF=Dilution Factor, A=Area of the chamber, D=Depth of the chamber, 106=conversion factor.
For the differential counts, a drop of blood was placed on a grease-free, clean slide. With the aid of a second slide, a thin film was made. This was allowed to air-dry. It was then fix with absolute Methanol and allowed to air-dry. The fixed thin smear was placed on a flat staining rack, covered with 10% Giemsa solution and allowed for 15mins. The stain was poured off, the slide gently washed in water, allowed to air-dry and examined microscopically using X 100 objective. The cells were counted using a leucocytes differential counting machine.
Statistical analysis
Significance differences among treatment groups were tested by one- way analysis of variance (ANOVA) and the comparison of the two mean values were made by Duncan’s multiple range tests. A significance level of p< 0.05 was used. The statistical analysis was performed by using the software programme IBM SPSS statistics (version 20).
Digestive enzyme assay of Clarias gariepinus fed with probiotics after 30, 50 and 70days of feeding experiment. The lipase levels were greater in fish fed diet supplemented with probiotics compared to control which was without probiotics. Single dose probiotics PRO1, PRO2 and PRO3 had better result than the double dose probiotics diets (diet supplemented with two species of bacteria in equal proportion) with highest value of amylase recorded in PRO2 diet containing Lactobacillus plantarum (Figures 1-3). Fish fed without probiotics showed a significant reduction of amylase (P<0.05) content in gut compared with fish incorporated with probiotics. Mean lipase and amylase values of 0.2824±0.11 and 0.0657±0.02, 0.2912±0.09 and 0.0651±0.01, 0.3421±0.13 and 0.0837±0.012 were recorded in the gut of fish fed with PRO2 (Lactobacillus plantarum) after 30, 50 and 70days of feeding experiment respectively. High amylase and lipase values obtained may be associated with better performance of the probiotic organisms as supported by different authors on some fish species: grouper Epinephelus coioides,11 Epinephelus bruneus12 and Nile tilapia (Oreochromis niloticus).13 After 30, 50 and 70days of feeding trial, the amylase levels of the fish fed with double dose probiotic diet (PRO4, PRO5 and PRO6) were significantly higher (P>0.05) than the control diet but lesser than single dose bacteria (PRO1, PRO2 and PRO3). The results obtained in this study was not in agreements with other work carried out by different authors Salinas et al.,14,15 reported the use of two different bacteria Lactobacillus delbrueckii lactis and Bacillus subtilis as double dose bacteria could be more effective and consistent than a monostrain or single dose probiotic. Improvement in the digestive enzymes activities may be related to the improvement in the microbial flora balance hence, increased digestion and enhanced absorption of food. Incorporation of these probiotics organisms into fish feed resulted in an increase in specific activity of amylase and lipase.
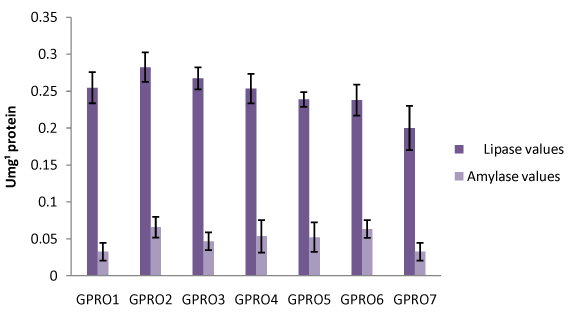
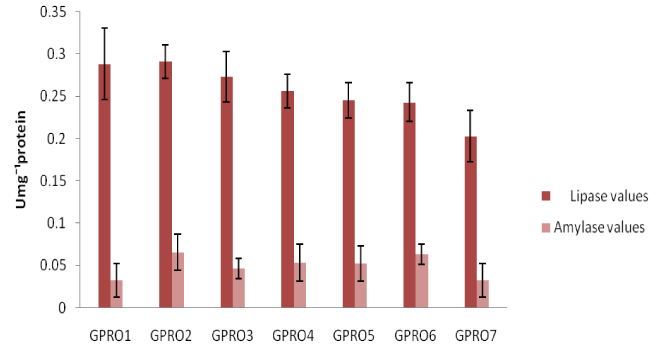
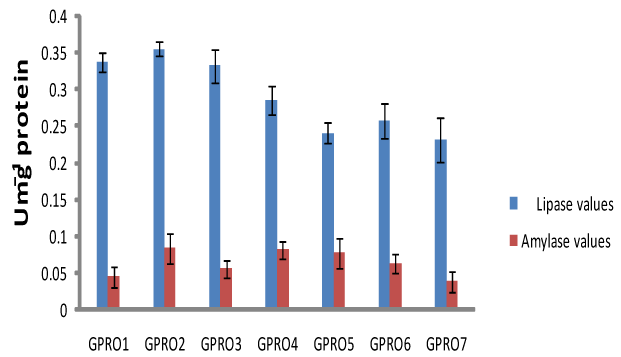
Haematological profile of Clarias gariepinus fed with probiotics after 30, 50 and 70days of feeding experiment
The number of white blood cell (WBC) in PRO2 was significantly higher in 30, 50 and 70days of the feeding experiment than the control and remaining group fed with probiotic with mean values of 6575±13.45/mm, 6800±13.41/mm and 7000/mm respectively (Tables 1-3). Relatively higher (P<0.05) number of white blood cell (WBC) were observed in the groups fed with probiotics. In this study, PRO2 recorded highest mean packed cell volume (PCV) values of 22.51±1.21%, 25.81±0.43% and 26.51±1.23in 30, 50 and 70days of feeding experiment respectively (P<0.05). Low PVC values were recorded in the double dose probiotic diets (PRO4, PRO5 and PRO6) compared with the single dose probiotic diets. The control experiment had higher PCV value of 20.75±3.34 at 30days and later reduced to 18.50±2.21 and 20.05±3.45 at 50 and 70days respectively. Low PCV recorded in the control experiment after 30 days may be associated with stress or probably the anemic condition of the fish as a result of no probiotics bacteria added to fight against diseases. An indication of low haematocrit (PVC %) targets on the anemic condition of fish and this situation occurs where the fish stop feeding as a result of any disease or stress.16 Haematological parameters can be used as an effective index to observe the physiological and pathological changes in fish to detect the fish health.17
Samples |
Packed cell vol. (%) |
White blood cell /mm3 |
Neutrophil (%) |
Lymphocytes (%) |
PRO1 |
22.12±1.23ª |
5600±17.21ᵃ |
18±3.3ª |
78±1.3ª |
PRO2 |
22.51±1.21ᵇ |
6575±13.45ᵃᵇ |
22±1.12ᵇ |
72±2.13ᵃᵇ |
PRO3 |
21.5±1.16ª |
2280±11.22ª |
27±2.11ª |
72±1.16ᵃᵇ |
PRO4 |
20.20±2.11ᵇ |
2220±16.26ᵇ |
29±1.87ª |
65±1.12ᵃᵇ |
PRO5 |
21.20±1.16ª |
2980±12.22ª |
15.5±1.24ª |
80.5±2.15ᵇ |
PRO6 |
18.5±2.24ª |
2000±24.12ª |
18.5±2.11ᵇ |
68.5±2.2ª |
PRO7 |
20.75±3.45ª |
2700±13.22ª |
15±1.09ᵇ |
70±2.14ª |
Table 1 Effect of probiotics on haematological factors of Clarias gariepinus after 30days of feeding experiment
Mean with the same superscript along columns are not significantly different (p≥0.05)
Samples |
Packed cell vol. (%) |
White blood cell /mm3 |
Neutrophil (%) |
Lymphocytes (%) |
PRO1 |
23.23±1.13ª |
5600±12.54ᵃ |
19±3.3ª |
80±1.3ª |
PRO2 |
25.81±0.43ᵇ |
6800±13.41ᵃᵇ |
23±1.12ᵇ |
73±2.11 |
PRO3 |
22.5±1.25ª |
2300±11.12ª |
28± 2.11ᵃᵇ |
75±2.1ᵃᵇ |
PRO4 |
21.25±1.23ª |
2250±14.25ᵃᵇ |
30±1.87ª |
69±1.12ᵃ |
PRO5 |
22.00±2.12ᵃᵇ |
3005±32.12ª |
15.5±1.24ª |
83.5±1.12ᵇ |
PRO6 |
19.5±2.21ª |
1800±14.22ª |
19.0±2.12ᵇ |
80.5±2.1ª |
PRO7 |
18.50±2.21ª |
2800±12.12ª |
17±2.09ª |
72±1.12ª |
Table 2 Effect of probiotics on haematological factors of Clarias gariepinus after 50days of feeding experiment
Mean with the same superscript along columns are not significantly different (p≥0.05)
Samples |
Packed cell vol. (%) |
White blood cell /mm3 |
Neutrophil (%) |
Lymphocytes (%) |
PRO1 |
25.11±1.23ª |
6000±12.54ᵃ |
19±3.3ª |
81±1.3ª |
PRO2 |
26.51±1.23ᵇ |
7000±13.45ᵃᵇ |
23±1.12ᵇ |
73±1.13 |
PRO3 |
24.51±1.25ª |
3325±21.12ª |
29± 2.11ª |
76±2.1ᵇ |
PRO4 |
22.25±1.14ª |
2350±16.26ª |
31±1.87ª |
69±1.12ᵃᵇ |
PRO5 |
22.25±2.12ᵇ |
3325±32.12ᵇ |
16.5±1.24ª |
83.5±1.16ᵇ |
PRO6 |
20.51±1.24ª |
2000±24.12ª |
19.5±2.11ᵇ |
80.5±2.2ª |
PRO7 |
20.05±3.45ª |
3200±13.22ª |
17±1.09ª |
83±2.11ª |
Table 3 Effect of probiotics on haematological factors of Clarias gariepinus after 70 days feeding experiment
Mean with the same superscript along columns are not significantly different (p ≥ 0.05).
Keys:
PRO1: Probiotic diet containing Lactobacillus brevis
PRO2: Probiotic diet containing Lactobacillus plantarum
PRO3: Probiotic diet containing Pediococcus pentosaceus
PRO4: Probiotic diet containing Lactobacillus
brevis1+Lactobacillus plantarum
PRO5: Probiotic diet containing Lactobacillus brevis+Pediococcus pentosaceus
PRO6: Probiotic diet containing Lactobacillus plantarum + Pediococcus pentosaceus
PRO7: Control diet without probiotics
The present investigation showed a significant improvement of the haematological status and the digestive enzymes (amylase and lipase) activities by the administration of probiotics to C. gariepinus diet compared to the control.
This study was carried out with financial support from Agricultural Research Council of Nigeria (ARCN) under Competitive Agricultural Research Grant Scheme (CARGS)–Grant Number RFA 2-37. We greatly acknowledge the support.
The author declares no conflict of interest.

©2014 Ayo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.