Advances in
eISSN: 2373-6402


Research Article Volume 7 Issue 4
1Department of Botany, University of Ibadan, Nigeria
2Cassava Transformation Agenda Project, International Institute of Tropical Agriculture, Nigeria
Correspondence: Chukwuka KS, Department of Botany, University of Ibadan, Ibadan-Nigeria
Received: July 24, 2017 | Published: August 22, 2017
Citation: Chukwuka KS, Okechukwu RU, Umukoro BO, et al. Arbuscular mycorrhiza fungi, NPK (15-15-15) and cow dung interaction in sustainable cassava production and food security. Adv Plants Agric Res. 2017;7(4):328-335. DOI: 10.15406/apar.2017.07.00262
Increasing human population coupled with the depletion and degradation of soil resources constitutes a threat to food security in sub-Saharan Africa. Sequel to this, the growth, performance and yield of cassava (Manihot esculenta L.) were assessed using pure culture of arbuscular mycorrhiza fungus - Glomus deserticola, NPK (15:15:15) and Cow dung singly and in combination with each other at four treatment levels under field conditions. Control experiment was also set up without any treatment. The experiment was factorial, in completely randomized block design and replicated four times. Cassava stem cuttings, 18 cm length were planted in well tilled soil at a distance of 1 m apart and allowed to grow for six months. During the study; plant height, stem girth, leaf area, leaf chlorophyll content and yield were assessed. At harvest, fresh and dry tuber weights were measured. Data collected were subjected to one way analysis of variance and means separated using Tukey’s HSD test (p≤0.05). The study showed that Glomus deserticola in combination with NPK produced cassava plants with significant differences (p<0.05) in height, stem girth, leaf area, chlorophyll content and yield. Arbuscular mycorrhiza fungus enhanced nutrient uptake of cassava plants. The balanced fertilization and amendment of the experimental soil with adequate nutrients supply provided useful agronomic information on the performance and yield of cassava.
Summary: Although some sub-Saharan African countries have for a number of year’s experienced significant agricultural success, demographic growth associated with high rates of erosion and land degradation continue to have an impact on food security in this part of the world. In this study, the growth and production of cassava (Manihot esculenta L.) was assessed by the contribution of pure culture of a mycorrhizal to arbuscules (Glomus deserticola), mineral fertilizers (NPK 15-15-15), cow manure and their combinations. The test was carried out on plots of 1 m × 1 m. On each plot, a cut of nearly 18 cm length was buried in the previously plowed soil. The experimental method consisted of a complete random block with a factorial plan of four repetitions. An equivalent number of control plots have not been processed. Vegetative growth parameters, including height of the plant, circumference of the stem and leaf surface were followed for six months, then the chlorophyll content of the leaves and yield were determined. These data were subjected to a simple variance analysis and a Tukey HSD test was performed to determine the significant differences between averages (p≤0.05). The use of Glomus deserticola in combination with NPK 15-15-15 mineral fertilizers has resulted in significant vegetative growth and yield significantly higher than other treatments. This suggests that this fungus improves the absorption of nutrients in cassava.
Keywords: soil fertilization, amendment, manihot esculenta, growth performance, food security
Cassava (Manihot esculenta Crantz) is a woody shrub that is widely cultivated in many tropical and subtropical regions of the world. It is propagated from stem cuttings and produces edible energy-rich tubers. Dry cassava tubers contain up to 90% carbohydrates, of which starch is dominant Montagnac et al.1 The importance of this crop in food security is reflected in its remarkably increasing production over the last decade. In 2014, about 270million tonnes constituting about 54% of global production were produced in Africa FAOSTAT.2 This crop is reputed for its remarkable tolerance to abiotic stresses due to its biochemical and physiological adaptations, intermediate C3-C4 photosynthetic pathway, reduction in leaf area index coupled with a relatively high stomatal sensitivity in response to water shortages and unaltered photosynthetic rates in nutrient-deficient soils El-Sharkawy.3 These characteristics make it suitable for both small and large-scale cultivation with moderate agricultural inputs. However, sustainable cassava production especially in the face of both increasing reliance on fertilizers and recurring environmental issues associated with their long-term use requires exploring alternative approaches that could help meet the food demands of rapidly expanding populations. The use of organic residues in combination with mineral fertilizers is a widely accepted soil fertility management strategy Edmeades,4 Diacono et al.5 Šimon et al.6 This practice is known to improve biological, chemical and physical properties of agricultural soils as opposed to the sole application of inorganic fertilizers Kotschi;7 Mulvaney et al.8 In fact, consistently lower cassava yields have been recorded under sole inorganic fertilizer application compared to when organic manures are combined with inorganic fertilizers (Ayoola and Adeniyan 2006; Ojeniyi et al.9 For example, Ojeniyi et al.9 reported that combination of 2.5t/ha of poultry manure and one quarter less the recommended amount of inorganic fertilizer (NPK 15-15-15; 600 kg/ha) led to twofold increase in yield of cassava compared to sole application of the same inorganic fertilizer at 600 kg/ha after 6 months of cultivation. Similarly, Islami et al., (2011) reported that the application of chemical fertilizers in monoculture cassava was inadequate to maintain sustained yields; but combining farm yield manure (FYM) with chemical fertilizers increased soil fertility and cassava production. Arbuscular Mycorrhizal Fungi (AMF) also referred to as Vesicular Arbuscular Mycorrhiza (VAM) are widespread in terrestrial ecosystems and form mutually beneficial associations with nearly 80% of higher plants Smith et al.10 They are characterized by their intercellular and intracellular growth forms in plant roots, which are referred to as vesicles and arbuscules Böhm et al.11 Mycorrhizal symbioses are known to mitigate the problem of efficient uptake of immobile nutrients by plants Bolan;12 Smith et al.13 Previous studies have shown that AMF are associated with salt Carretero et al.14 And drought tolerance Qiangsheng et al.;15 Ruiz-Lozano et al.16 and cassava is known to thrive under these conditions. The potentials of AMF in sustainable crop production have been demonstrated by many scholars Li et al.17 Céli et al., 2016; Tchabi et al.18 However its applicability has not been fully addressed.
Cassava tolerates low soil nutrient levels. However, this crop needs substantial fertilization to attain high yields like many other food crops Howeler.19 Perhaps, cassava would not have been a successful crop under both fertilized and un-fertilized conditions without its dependency on mycorrhizal fungi for nutrient uptake, especially phosphate uptake Habte et al.20 These authors reported cassava mycorrhizal dependency (that is, the change in cassava growth due to arbuscular mycorrhizal colonization) of 60% in contrast to the much lower values (44-46%) obtained in many crop species Tawaraya.21 Recently, Burns et al. (2012) showed that mycorrhizal dependency of cassava could reach up to 93%. This dependency is indicative of the wide variety of AMF associated with cassava roots as Glomus, Gigaspora and Acaulospora which are the most commonly reported genera Straker et al.;22 Bi Voko et al.;23 dos Santos Heberle et al.;24 Begoude et al.25 Several species in the genus Glomus including G. manihotis Sieverding et al.26 G. fasciculatum, G. clarum et al.27 G. deserticola Okon et al.,28 G. intraradices Carretero et al.;29 G. aggregatum20 and G. etunicatum Salami et al.,30 have been tested in field studies with or without fertilization. Obviously, cassava’s poor root system architecture, a factor that is crucial in nutrient uptake and subsequent productivity in many crops Smith et al.10
Is a major cause of its dependency on mycorrhiza for nutrient acquisition. The beneficial effects of AMF association with cassava have been the subject of several studies. Howeler et al.,31 Reported a significant increase in growth and dry matter content of mycorrhiza-inoculated cassava cuttings compared to non-inoculated plants, which were P-deficient even at high P soil levels, Sieverding et al.,26 Found that N-P-K concentration ratios in cassava shoots and roots were more balanced in mycorrhiza-inoculated plants than in non-inoculated plants. In the same vein, Ceballos et al. (2013) showed that not only 20% increase in cassava yield was obtained due to addition of Rhizophagus irregulars but also a 50% reduction in phosphate fertilizer. Other beneficial effects have been reported including enhanced plantlet survival, shoot, root and tuber formation Azcón-Aguilar et al.;32 salt damage alleviation Carretero et al.29 and resistance to transplant stress Carretero et al.14 Given the growing body of evidence on the beneficial effects of AMF on cassava, the present study tested effects of AMF and its combination with organic and inorganic fertilizers to improve cassava productivity in a sustainable manner. Therefore this study hypothesized that soil inoculated with Glomus deserticola will enhance cassava growth.
Study area
This study was carried out at the Botany Research Farm, Department of Botany, University of Ibadan (7o26.44' N, 3053.76' E) between 18 July 2016 and 18 January 2017. The University has mean annual rainfall and temperature of 1316 mm and 27.6oC respectively.
Soil and cow dung analyses
Soil samples were randomly taken from 0-20cm depth before planting, bulked, air-dried and sieved using 2mm sieve for analysis. The particle size analysis was done by pipette method Gee et al.33 Soil pH in water was determined using soil: water ratio of 1:2 with a glass electrode pH meter. Organic carbon was determined using Walkey and Black method (Nelson and Sommers, 1996). Total nitrogen (N) in the soil was determined by Kjedahl digestion Bremner.34 Exchangeable bases in the samples were extracted in 1M NH4 OAC at pH 7.0. Calcium (Ca) and magnesium (Mg) in the extract were read by atomic absorption spectrophotometer (AAS). Sodium (Na) and potassium (K) were analyzed by flame photometry. Available phosphorus (P) was determined by Bray-1 extraction and determined colourimetrically by the molybdenum blue procedure Bray et al.35
Cow dung samples were air-dried and ground to powder and analysed with wet digestion method using 5:1:1 ml of HNO3: H2SO4:HClO4 acid. Total N was determined by micro–Kjeldahl method (Jackson, 1962). For P, K, Ca and Mg, samples (0.5g) were ashed, dissolved in 10% hydrogen chloride (HCl) and diluted to 50 ml. Phosphorous was determined using vandal molybdate colorimetric. Calcium and Magnesium were determined by EDTA titration while Na and K by flame photometry. The physico-chemical properties of both soil and cow dung used were analyzed at the Department of Agronomy, University of Ibadan.
Experimental design and treatment application
The experiment involved seven treatments: AMF (Glomus deserticola) 20, 30, 40 and 50g), NPK 15:15:15 40, 60, 80 and 100g; cow dung 200, 300, 400 and 500g; AMF and NPK 20+40, 30+60, 40+80 and 50+100g, AMF and cow dung 20+200, 30+300, 40+400, 50+500g, cow dung and NPK 40+200, 60+300, 80+400, 100+500g laid out in a completely randomized design (CRD) with four replicates. The soil was tilled before planting. Cassava stem cuttings (TME 419) of about 15cm long were planted horizontally and buried completely at 5cm depth in heaps of soil at a planting distance of 1m apart in a plot size of 14m×14m. The treatments were applied two weeks after establishment. Application of NPK 15:15:15, cow dung and Glomus deserticola was done using the methods of Ojeniyi et al.,9 Mathias et al.,36 and Okon et al.28 Respectively, four different levels of each G. deserticola (20g, 30g, 40g and 50g); NPK 15:15:15 (40g, 60g, 80g and 100g) and cow dung (200g, 300g, 400g and 500g) were applied both singly and in combination around the growing stem cuttings.
Data collection
Data collection commenced two weeks after application of treatments and subsequently forth nightly for five months. The following growth parameters: plant height, stem girth and leaf area were determined forth nightly. Plant height (cm) and leaf area (cm2) were determined using a Measuring Tape and Portable Electronic Area Metre Model Li-3000 respectively. Stem girth was measured at 5cm above heap level using Mitutoyo Digimatic Electronic calliper (MDEC) Model CD-8″P. The leaf chlorophyll content was determined by using the central leaf from freshly excised leaves of the same age. The excised leaf was weighed, cut into smaller pieces and stored in the dark for 24hrs in a mixture of 10 ml of 95% ethanol and 99.5% acetone (1:1v/v). One millilitre of the concentrated leaf extract was added to a cuvette and adjusted to 5ml with the ethanol-acetone mixture. The Absorbance of the mixture was measured spectrophotometrically at 652nm using a Unico spectrophotometer. This value was taken as reference by resetting the absorbance to zero and measuring that of the diluted leaf extract. Chlorophyll concentration (ml/g) was estimated using equation (1)
Chlorophyll Concentration =
Where A652=Sample OD value (Absorbance at 652 nm)
V=Volume of the sample (ml)
W=Weight of the sample (g)
The yield data was collected six months after planting and these include fresh and dry weight of tubers. The plants were harvested and separated into roots and stems. Dry weight was determined after oven drying at 1000C for 48hours using Ohaus Sensitive Electronic Digital Weighing Balance Model SPX2202.
Data analysis
Data were analysed using Minitab 16 Statistical Software (2010). A one way Analysis of Variance (ANOVA) was carried out to test the effects of the treatments on cassava growth and development. Means were separated using Tukey’s HSD test (p≤0.05).
(Table 1) shows the values of the soil physicochemical properties of the experimental site and the nutrient composition of cow dung used in the study. The soil was silt loamy and slightly acidic. Total nitrogen and Organic carbon were higher than 0.11% and 2% respectively, which are critical values for Nigerian soils Adepetu.37 The soil was poor in Phosphorus (Available P < 10 mg/Kg) while potassium was detected in adequate amounts (Exchangeable K > 0.2 C mol (+)/Kg. Effective CEC (sum of equivalent charge concentrations of cations (Ca2+, Mg2+, K+ Na+ and Al3+) was low (i.e between 5-15 C mol (+)Kg-1),Cassava plant height (cm) The study showed significant differences in plant height from 8 weeks after establishment (WAE). Between 8 to 12WAE, three main effects were distinguished. AMF and NPK combination (30 g of AMF and 60 g of NPK) significantly enhanced plant height (133 ± 8, 145 ± 10 and 149 ± 13 cm respectively). On the other hand, sole application of cow dung did not produce plants with appreciable increase in height (57 ± 9, 71 ± 8 and 70 ± 14 cm respectively). Other treatments produced plant heights similar to the control. On the other hand, from 14WAE to 20WAE, the plants showed decreasing performance in height in the order AMFNPKL2 (30 + 60) g > NPK 100 g and CDNPKL4 (500 + 100) g > control > CD (400 g) > CD (200g) (Table 2).
Parameter |
Soil |
Cow Dung |
pH |
5.86 |
- |
Nitrate |
4.66 g/Kg |
1.81% |
Organic carbon |
54.75 g/Kg (H) |
- |
Phosphate |
- |
0.53% |
Average phosphorus |
6.52 mg/Kg (L) |
- |
Exchangeable aluminium |
0.5 C mol (+)/Kg |
- |
Calcium |
8.86 C mol (+)/Kg |
0.28% |
Magnesium |
0.69 C mol (+)/Kg |
0.14% |
Potassium |
0.55 C mol (+)/Kg |
1.04% |
Sodium |
0.18 C mol (+)/Kg |
0.22% |
Manganese |
17.9 mg(+)/Kg |
2160 mg(+)/Kg |
Iron |
224 mg(+)/Kg |
17.5 mg(+)/Kg |
Copper |
1.34 mg(+)/Kg |
15.05 mg(+)/Kg |
Zinc |
7.83 mg(+)/Kg |
1625 mg(+)/Kg |
Silt |
1560 g/Kg |
- |
Clay |
116 g/Kg |
- |
Sand |
728 g/Kg |
- |
Table 1 Physico-chemical characteristics of the soil at the experimental site, Botany Research Farm, University of Ibadan and chemical composition of cow dung
Treatment |
8WAE |
10WAE |
12WAE |
14WAE |
16WAE |
18WAE |
20 WAE |
Control |
97.33±19.66ab |
100.10±16.42ab |
122.42±5.47ab |
128.98±2.37abc |
128.55±1.61ab |
128.62±1.40abc |
131.15 ± 2.38abc |
AMF 20 g |
85.55±16.66ab |
94.70±15.66ab |
100.72±17.79ab |
108.35±16.38abc |
114.18±14.93ab |
114.25±13.64abc |
115.08 ± 12.35abc |
AMF 30 g |
64.98 ± 5.46ab |
75.08±6.74ab |
82.70±6.28dab |
91.78±5.26abc |
89.43±5.76ab |
86.45±6.35abc |
86.58 ± 6.21abc |
AMF 40 g |
83.90±7.18ab |
91.65±6.08ab |
93.25±7.13ab |
93.13±8.05abc |
93.70±7.18ab |
93.60±5.71abc |
98.90 ± 7.02abc |
AMF 50 g |
70.10±16.37ab |
78.38±14.60ab |
91.13±11.52ab |
97.50±9.30abc |
90.78±6.00ab |
99.30±9.55abc |
107.22 ± 11.06abc |
NPK 40 g |
103.12±10.08ab |
113.32±10.78ab |
130.33±8.26ab |
132.77±7.48abc |
134.90±18.46ab |
136.67±8.05abc |
137.90 ± 7.60abc |
NPK 60 g |
113.38± 8.94ab |
90.80±8.78ab |
121.57±5.48ab |
120.03±6.07abc |
122.23±6.49ab |
124.07±7.34abc |
124.47±4.30abc |
NPK 80 g |
91.75±14.52ab |
115.92±10.7 b |
106.78±14.33ab |
108.50±13.27abc |
99.10±6.49ab |
99.03±7.34abc |
115.12±13.68abc |
NPK 100 g |
120.85±1.74ab |
130.52±20.18ab |
133.22±22.37ab |
138.45±20.42ab |
136.95±19.88ab |
137.70±17.62ab |
143.05± 20.88ab |
CD 200 g |
57.35±8.90b |
70.90±7.55b |
69.75±14.08b |
65.15±20.68c |
66.60±19.64b |
65.85±18.86c |
63.70 ± 18.96c |
CD 300 g |
63.50±14.82ab |
73.08±15.03b |
78.60±12.95ab |
88.35±8.99abc |
88.18±6.10ab |
87.32±5.19abc |
88.73 ± 7.25abc |
CD 400 g |
64.58±6.31ab |
69.98±7.58b |
71.63±8.99b |
84.40±8.40bc |
89.68±10.38ab |
74.33±5.79bc |
72.08 ± 7.43bc |
CD 500 g |
79.60±6.42ab |
90.25±9.31ab |
98.45±14.98ab |
102.15±16.03abc |
105.68±16.75ab |
107.32±16.47abc |
110.65 ± 16.54abc |
AMFNKPL1 |
97.15 ± 13.21ab |
97.78±13.24ab |
108.93±14.98ab |
114.47±10.19abc |
116.43±12.93ab |
118.97±13.43abc |
124.13 ± 13.81abc |
AMFNKPL2 |
133.22 ± 7.95a |
145.42±9.62a |
148.92±12.81a |
159.60±13.09a |
158.87±12.95a |
149.70±8.24a |
165.73 ± 11.96a |
AMFNKPL3 |
75.90 ± 7.87ab |
106.10±9.38ab |
118.70±7.81ab |
123.77±7.44abc |
121.70±7.83ab |
120.45±8.18abc |
121.12 ± 9.86abc |
AMFNKPL4 |
97.38 ± 12.54ab |
110.45±13.04ab |
118.72±12.94ab |
124.45±11.46abc |
104.42±18.63ab |
118.35±11.84abc |
110.28 ± 13.41abc |
AMFCDL1 |
81.73 ± 15.11ab |
91.15±13.24ab |
99.33±13.77ab |
102.55±13.54abc |
109.95±14.60ab |
111.62±14.23abc |
112.72 ± 14.61abc |
AMFCDL2 |
91.05 ± 12.50ab |
102.48±12.14ab |
108.82±16.98ab |
107.12±10.78abc |
95.43±4.69ab |
100.09±6.01abc |
96.28 ± 9.37abc |
AMFCDL3 |
102.85±15.11ab |
112.18±12.87ab |
106.48±17.65ab |
115.55±18.14abc |
118.70±14.52ab |
122.50±16.97abc |
130.30 ± 24.30abc |
AMFCDL4 |
66.40 ± 15.33ab |
76.23±15.36ab |
89.45±11.83ab |
91.90± 9.18abc |
90.48±8.55ab |
89.00 ± 7.56abc |
92.83 ± 10.37abc |
CDNPKL1 |
95.23 ± 8.75ab |
104.73±9.77ab |
113.22±14.85ab |
121.00±16.82abc |
116.08±20.04ab |
118.50±19.56abc |
122.00 ± 21.29abc |
CDNPKL2 |
99.33 ± 12.54ab |
111.02±12.71ab |
126.52±13.31ab |
120.40±12.09abc |
119.43±9.71ab |
123.40±11.44abc |
128.10 ± 12.59abc |
CDNPKL3 |
83.45± 22.92ab |
99.95±22.53ab |
110.88±23.01ab |
126.88±14.89abc |
123.38±21.13ab |
121.80±1.01abc |
117.40 ± 21.66abc |
CDNPKL4 |
110.93±14.44ab |
122.83±10.99ab |
136.37±6.00ab |
140.80±4.32ab |
115.50±3.88ab |
115.37±3.86abc |
122.67 ± 1.68abc |
Table 2 Effects of treatments on cassava plant height (cm)
Means and standard error of the treatments separated using Tukey’s HSD (p < .05). Means with the same letter along the column are not significantly different.
AMF: Arbuscular mycorrhiza fungi;
CD: Cow dung;
Cassava leaf area (cm2)
There was a general increase in leaf area from 8 -12WAE. However, from 14 - 20WAE, the leaf area showed general decrease in size. On the other hand, there were significant differences at 8 and 10WAE among treatments for plants with AMF and NPK combination (30 + 60 g) having the widest leaf areas while plants treated with cow dung (400 g) showed the least leaf area. Although at 12WAE and 16 -20WAE, no significant differences among treatments were observed. It is important to note that the interaction of AMF with any combination of treatment produced plants with big leaf areas (Table 3).
Treatment |
8WAE |
10WAE |
12WAE |
14WAE |
16WAE |
18WAE |
20WAE |
Control |
150.23 ± 9.99ab |
253.65±62.12ab |
280.65 ±67.30a |
198.10 ±26.53ab |
90.16±5.67a |
75.25 ± 17.12a |
71.45 ± 7.58a |
AMF 20 g |
158.90±10.96ab |
277.86±39.99ab |
146.90±33.29a |
164.88±20.30ab |
99.39 ± 5.49a |
98.79 ± 11.30a |
57.99 ± 11.01a |
AMF 30 g |
118.91±14.45ab |
164.61±29.83ab |
146.96±25.50a |
135.01 ± 5.23ab |
84.34 ± 7.13a |
65.27 ± 13.68a |
48.41 ± 17.48a |
AMF 40 g |
126.06 ± 6.70ab |
240.28±15.85ab |
170.95±25.77a |
119.51±13.69b |
73.40±4.60a |
46.49 ± 6.35a |
74.43 ±7.66a |
AMF 50 g |
132.01±14.00ab |
215.10±28.02ab |
181.17±10.42a |
157.34 ± 8.30ab |
95.58 ± 9.99a |
68.06 ± 16.36a |
74.77 ± 8.82a |
NPK 40 g |
152.34±23.37ab |
323.27±41.12ab |
283.57±56.37a |
178.63±54.82ab |
109.57 ± 5.26a |
80.21 ± 18.31a |
85.42 ± 3.12a |
NPK 60 g |
174.94±26.22ab |
334.26±9.38ab |
225.37±46.52a |
140.24±35.05ab |
87.65 ± 4.53a |
95.16±4.99a |
74.11 ± 4.50a |
NPK 80 g |
140.68 ± 6.68ab |
276.43±61.43ab |
245.76±69.22a |
208.81±49.33ab |
69.34± 11.65a |
45.72±17.23a |
43.62 ± 12.44a |
NPK 100 g |
180.17±20.54ab |
341.05±16.60ab |
307.98±49.49a |
245.45±40.19ab |
85.77 ± 9.87a |
71.65 ± 23.80a |
72.75 ± 23.57a |
CD 200 g |
120.87±16.42ab |
166.22±28.63ab |
108.36±32.44a |
125.38±24.52ab |
96.77 ± 15.70a |
68.66 ± 9.95a |
88.69 ± 15.94a |
CD 300 g |
138.59±28.29ab |
217.11±50.51ab |
193.75±26.94a |
149.18±11.41ab |
96.88 ± 5.02a |
85.11 ± 14.20a |
48.91 ± 11.60a |
CD 400 g |
110.31± 10.26b |
163.34±27.45b |
127.84±16.86a |
131.16 ±10.94ab |
88.70 ± 11.65a |
66.74 ± 14.41a |
63.44 ± 19.18a |
CD 500 g |
152.62±18.10ab |
217.10±36.35ab |
203.99±47.20a |
134.81 ±19.10ab |
91.51 ± 13.93a |
65.51 ± 7.07a |
102.22 ± 32.77a |
AMFNKPL1 |
186.48±13.92ab |
251.47±39.32ab |
182.11±20.21a |
169.85±13.38ab |
71.92 ± 2.46a |
61.71 ± 18.25a |
82.02 ± 2.31a |
AMFNKPL2 |
227.50±30.98a |
405.18±34.67a |
349.06±39.69a |
341.50 ± 1.45a |
61.8 ± 39.0a |
59.83 ± 28.26a |
61.38 ± 16.74a |
AMFNKPL3 |
161.87±12.57ab |
284.93±38.60ab |
215.56±33.08a |
184.55 ± 9.70ab |
83.04 ± 6.23a |
61.36 ± 15.47a |
71.59 ± 11.56a |
AMFNKPL4 |
159.06±1.98ab |
228.64±53.12ab |
233.76±39.67a |
212.17±16.16ab |
113.57 ± 6.70a |
49.92 ± 14.32a |
63.39 ± 19.99a |
AMFCDL1 |
131.67±21.99ab |
212.86±59.60ab |
175.14±64.11a |
188.33±66.55ab |
104.90 ± 7.60a |
89.51 ± 14.12a |
62.28 ± 12.71a |
AMFCDL2 |
175.92±20.74ab |
227.97±51.84ab |
205.86±52.05a |
137.08±28.09ab |
76.49 ± 8.02a |
58.97 ± 6.78a |
33.44 ± 8.43a |
AMFCDL3 |
172.03±14.94ab |
282.84±57.02ab |
237.23±69.31a |
217.81±46.00ab |
81.87 ± 21.65a |
95.27 ± 9.39a |
73.75 ± 13.04a |
AMFCDL4 |
138.90±20.62ab |
208.48±37.82ab |
190.26±33.90a |
132.88±12.73ab |
79.42 ± 5.86a |
64.16 ± 10.15a |
60.45 ± 4.87a |
CDNPKL1 |
158.05 ± 6.91ab |
308.21±19.76ab |
242.42±49.45a |
205.83±62.75ab |
83.41 ± 14.06a |
70.43 ± 13.35a |
49.98 ± 16. 07a |
CDNPKL2 |
154.62±19.24ab |
322.37±60.63ab |
272.03±59.67a |
150.32±10.35ab |
96.22 ± 19.71a |
111.28±24.52a |
101.01 ± 18.26a |
CDNPKL3 |
151.10±52.28ab |
280.98±56.74ab |
287.94±72.75a |
224.69±31.31ab |
104.38 ± 3.22a |
110.80±15.63a |
90.44 ± 4.04a |
CDNPKL4 |
212.93±25.73ab |
323.77±64.52ab |
353.08±44.78 a |
247.58±20.48ab |
85.15 ± 2.38a |
84.71 ± 16.00a |
82.76 ± 12.94a |
Table 3 Effects of treatments on cassava leaf area (cm2)
Means with the same letter along the column are not significantly different.
AMF: Arbuscular mycorrhiza fungi;
CD: Cow dung;
WAE: Weeks After Establishment.
Cassava stems girth (mm)
Significant differences in plant stem girth were observed from 10-20 WAE. The combination of AMF and inorganic fertilizer (30 g of AMF and 60 g of NPK) produced plants with the largest stem girth while plants treated with cow dung (200 g) produced the least girth. Stem girth showed similar growth pattern throughout the period of study (Table 4).
Treatment |
10WAE |
12WAE |
14WAE |
16WAE |
18WAE |
20WAE |
Control |
14.83 ± 1.89ab |
17.13 ± 0.62ab |
17.08 ± 0.44ab |
17.48 ± 0.60ab |
17.48 ± 0.64ab |
17.10 ± 0.54ab |
AMF 20 g |
14.97 ± 1.53ab |
15.40 ± 1.75ab |
16.24 ± 1.75ab |
15.87 ± 1.61ab |
15.67 ± 2.17ab |
16.16 ± 1.29ab |
AMF 30 g |
12.55 ± 0.88b |
12.80 ± 0.69ab |
12.46 ± 0.57ab |
13.10 ± 0.60ab |
12.78 ± 0.80ab |
12.76 ± 0.63ab |
AMF 40 g |
15.40 ± 1.06ab |
15.06 ± 0.97ab |
15.36 ± 0.57ab |
15.20 ± 1.05ab |
15.37 ± 0.38ab |
15.39 ± 0.60ab |
AMF 50 g |
13.12 ± 1.92ab |
13.81 ± 1.90ab |
14.85 ± 1.20ab |
13.94 ± 1.08ab |
14.68 ± 1.28ab |
13.66 ± 1.04ab |
NPK 40 g |
17.15 ± 1.22ab |
17.90 ± 0.98ab |
17.99 ± 2.15ab |
18.45 ± 1.23ab |
18.66 ± 1.16ab |
18.09 ± 1.50ab |
NPK 60 g |
17.01 ± 0.86ab |
17.04 ± 0.43ab |
17.92 ± 0.70ab |
18.13 ± 0.65ab |
18.07 ± 0.67ab |
17.70 ± 0.14ab |
NPK 80 g |
15.53 ± 1.51ab |
14.78 ± 1.45ab |
15.09 ± 1.27ab |
13.42 ± 0.75ab |
14.69 ± 1.37ab |
14.94 ± 1.25ab |
NPK 100 g |
17.33 ± 1.98ab |
17.25 ± 2.05ab |
17.53 ± 2.25ab |
18.14 ± 2.05ab |
17.39 ± 2.09ab |
17.51 ± 2.21ab |
CD 200 g |
11.83 ± 1.00b |
12.32 ± 1.44b |
10.72 ± 2.48b |
10.92 ± 2.26b |
10.70 ± 2.18b |
10.28 ± 2.19b |
CD 300 g |
13.14 ± 1.21ab |
13.95 ± 1.02ab |
13.85 ± 1.26ab |
14.46 ± 0.68ab |
14.46 ± 0.57ab |
14.39 ± 0.68ab |
CD 400 g |
13.02 ± 1.29ab |
12.47 ± 1.28b |
13.32 ± 1.66ab |
13.01 ± 1.43ab |
12.45 ± 1.65b |
12.82 ± 1.53ab |
CD 500 g |
14.99 ± 0.63ab |
15.01 ± 0.83ab |
15.84 ± 1.17ab |
16.16 ± 1.28ab |
15.93 ± 1.19ab |
15.14 ± 1.64ab |
AMFNKPL1 |
16.57 ± 2.38ab |
16.31 ± 2.05ab |
16.51 ± 2.13ab |
17.40 ± 1.63ab |
16.65 ± 1.70ab |
16.42 ± 1.99ab |
AMFNKPL2 |
20.72 ± 1.61a |
20.14 ± 1.43a |
20.76 ± 1.80a |
21.08 ± 1.89a |
21.19 ± 1.56a |
20.87 ± 1.93a |
AMFNKPL3 |
16.27 ± 1.70ab |
17.61 ± 1.00ab |
17.69 ± 0.87ab |
17.86 ± 0.88ab |
17.71 ± 0.69ab |
17.84 ± 0.69ab |
AMFNKPL4 |
15.77 ± 1.06ab |
16.54 ± 1.18ab |
16.53 ± 1.06ab |
16.71 ± 0.99ab |
17.06 ± 1.17ab |
16.16 ± 1.24ab |
AMFCDL1 |
14.09 ± 1.68ab |
13.90 ± 1.77ab |
15.34 ± 1.55ab |
14.73 ± 2.12ab |
14.32 ± 1.63ab |
14.50 ± 1.61ab |
AMFCDL2 |
15.10 ± 1.15ab |
15.27 ± 0.80ab |
14.85 ± 1.08ab |
14.63 ± 0.79ab |
15.75 ± 0.72ab |
14.57 ± 1.22ab |
AMFCDL3 |
16.09 ± 1.08ab |
15.62 ± 1.94ab |
15.80 ± 1.82ab |
16.54 ± 1.86ab |
16.38 ± 1.73ab |
15.78 ± 2.31ab |
AMFCDL4 |
13.67 ± 1.64ab |
14.11 ± 1.20ab |
13.64 ± 0.69ab |
13.79 ± 0.68ab |
13.82 ± 0.78ab |
13.86 ± 0.94ab |
CDNPKL1 |
16.54 ± 0.90ab |
16.91 ± 1.46ab |
17.17 ± 1.74ab |
17.41 ± 1.86ab |
17.52 ± 1.97ab |
17.58 ± 2.15ab |
CDNPKL2 |
16.65 ± 1.22ab |
17.40 ± 1.23ab |
16.69 ± 0.81ab |
16.74 ± 0.85ab |
17.75 ± 2.46ab |
16.71 ± 0.97ab |
CDNPKL3 |
16.27 ± 2.60ab |
16.68 ± 2.44ab |
15.88 ± 2.43ab |
17.38 ± 2.15ab |
16.97 ± 2.09ab |
17.34 ± 2.15ab |
CDNPKL4 |
18.32 ± 1.21ab |
18.47 ± 0.52ab |
19.29 ± 0.65a |
14.09 ± 2.11ab |
16.76 ± 2.94ab |
15.99 ± 2.83ab |
Table 4 Effects of treatments on cassava stems girth (mm)
Means and standard error of the treatments separated using Tukey’s HSD (p < .05). Means with the same letter along the column are not significantly different.
AMF: Arbuscular mycorrhiza fungi;
CD: Cow dung;
WAE: Weeks after Establishment.
Cassava leaf chlorophyll content (ml/g)
The effects of different treatments on the leaf chlorophyll content at 10 weeks after planting and establishment of the plants are represented in Figure 1. The mean chlorophyll concentration was highest in the combined application of inorganic fertilizer and G. derserticola (0.1825 ± 0.0007 ml/g) and lowest with cow dung in combination with G. derserticola. Chlorophyll in plants treated with sole NPK was next (0.15497 ± 0.000246 ml/g) after the combined application of G. deserticola and NPK. Cow dung, G. deserticola and the control treatments had similar effect on leaf chlorophyll content with concentrations of 0.0909 ± 0.0006, 0.0922 ± 0.0005 and 0.0925± 0.0004 ml/g respectively (Figure 1).
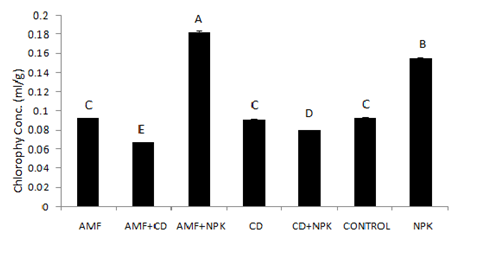
Figure 1 Effects of treatment on the cassava leaf chlorophyll content (ml/g).
AMF: Arbuscular Mycorrhiza Fungi
CD: Cow Dung
NPK: Inorganic Nitrogen: Phosphorus: Potassium fertilizer (15:15:15).
Yield
(Figure 2) represents the fresh cassava weight obtained from the different treatments. Significant differences were observed among the treatments. As in other growth parameters, the highest yield was obtained in cassava treated with the mixture of 30 g of G. deserticola and 60 g of NPK (0.87 ± 0.07 Kg) while the lowest yield was observed in all the cow dung treatments. The control (0.23 ± 0.05 Kg) was comparable to all other treatments. The figure also show that dry weight of the tubers follow the same pattern as the fresh weight with the combined AMF-NPK (30 g+60 g) producing the highest dry weight (0.17± 0.01 Kg) while cow dung (300 g) alone produced the lowest tuber dry weight (0.008±0.006 Kg).
Several researchers have demonstrated the beneficial effects of mycorrhizal inoculation on growth and yield of cassava Douds et al.;38 Carretero et al.;14 Séry et al.39 However, the combination of AMF and organic/inorganic fertilizers has not been adequately investigated. The results of this study suggest that G. deserticola inoculation in combination with inorganic fertilizer application at the rate of (30g and 60g respectively) has beneficial effects on all the growth parameters studied. In this study, leaf chlorophyll content was a good indicator of growth and this was reflected in the yield of the cassava plants studied. Ekanayake et al.,24 reported that soil inoculated with 10g of Glomus clarum and G. mosseae enhanced chlorophyll production in young cassava plants, with the former species supporting more chlorophyll synthesis than the later. Howeler et al.,31 reported an increase in cassava growth under different combinations of P input with AMF as opposed to the sole application of AMF. Most of the soils supporting cassava cultivation in the south-western region of the Nigeria are P deficient thereby underscoring the need for strategies for sustainable soil fertility techniques Salami et al.40 The physico-chemical properties of the experimental soil do not differ considerably from those reported by these authors.
Arbuscular mycorrhiza fungi are crucial component of the soil ecosystem that enhances nutrient uptake and absorption Bolan,12 The increase in growth attributes recorded in this study could be as a result of these processes. Similarly, Sieverding et al.,26 opined that mycorrhiza inoculation enhanced nutrient uptake in the shoot of cassava compared to non-inoculated plants. In this study, G. deserticola inoculation enhanced the growth and yield of cassava therefore supports reduced inorganic fertilizers inputs. This result compares favourably with previous studies on the effects of mycorrhizal inoculations on cassava growth. Ceballos et al. (2013) showed that Rhizophagus irregularis inoculation produced 20% increase in cassava yield and 50% reduction in phosphate fertilizer. Similarly Sridevi et al.,41 studying the response of cassava to Glomus fasciculatum inoculation at increased NPK levels reported that yield attributes, like number of tubers, tuber yield were optimal under increased NPK and AMF application. In the present study, inoculation of G. deserticola enhanced the performance and yield the cassava plants studied.42–44
The cassava variety TME 419 used in this study responded positively to G. deserticola inoculation in combination with inorganic fertilization. This finding indicates the potentials of arbuscular mycorrhiza fungi as a biological agent for sustainable agriculture.
None.
The author declares no conflict of interest.

©2017 Chukwuka, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.