Advances in
eISSN: 2373-6402


Research Article Volume 8 Issue 6
Faculty of Animal Sciences and Agricultural Technology, Silpakorn University, Thailand
Correspondence: Suphavadee Chimtong, Faculty of Animal Sciences and Agricultural Technology, Silpakorn University, Phetchaburi IT Campus, Sampraya, Cha-am, Phetchaburi, Thailand
Received: January 27, 2018 | Published: November 16, 2018
Citation: Chimtong S. Antibacterial activity of chito-oligosaccharides (COSS) from shrimp shells wastes. Adv Plants Agric Res. 2018;8(6):392-394. DOI: 10.15406/apar.2018.08.00356
Shrimp shells wastes are chitin source. The aim of this study was to investigate the antibacterial effect of chito-oligosaccharides (COSs) prepared from three shrimp shells that were Pacific white shrimp shells (Litopenaeus vannamei; COS1), Giant tiger prawn shells (Penaeus monodon; COS2) and Giant freshwater prawn shells (Macrobrachium rosenbergii; COS3). Chitin was isolated from shrimp shells wastes by chemical treatments, chitosan and COSs were prepared by deacetylation and acid hydrolysis, respectively. Thin-layer chromatography (TLC) was used to detect COSs that appeared four major sizes of COSs in all samples. The COSs were used to study the inhibitory effect against the growth of three bacteria: Vibrio parahaemolyticus, Escherichia coli and Pseudomonas aeruginosa. The result showed that COS1 and COS3 presented a highest inhibitory effect against the growth of V. parahaemolyticus that were 61.75% and 89.40%, respectively whereas COS2 inhibited similarity growth of E. coli and V. parahaemolyticus (34.44% and 36.87%, respectively). However, all COSs exhibited a bactericidal effect on all bacteria tested but E. coli and P. aeruginosa were inhibited lower than 50%. These present suggest that COS3 was prepared from Giant freshwater prawn shells suitable to use as antibacterial agent.
Keywords: chito-oligosaccharide, shrimp shells, vibrio parahaemolyticus, escherichia coli, pseudomonas aeruginosa
COSs, chito-oligosaccharides; MIC, minimum inhibitory concentration; C, numbers of optical density as the control; T, numbers of optical density obtained from each tested sample
Chitin is a linear polysaccharide consisting of β,1,2 linked N-acetyl-D-glucosamine residues. It is often considered the second most abundant polysaccharide in nature and occurs mainly as a structural component in the cell walls of fungi and yeasts and in the exoskeletons of insects and arthropods (e.g., crabs, lobsters and shrimps). Chitin is insoluble in water and exists mainly in two crystalline polymorphic forms, α and β3 besides, chitin can be converted to chitosan by enzymatic preparations or chemical process.2,4 Chemical methods are used extensively for commercial purpose of chitosan preparation because of their low cost and suitability to mass production.4 The antimicrobial activity of chitosan has been recognized against some kinds of microorganisms. It is generally recognized that chitosan with a high degree of deacetylation has high antimicrobial activity.5 However, chitosan showed antibacterial activity only in acidic medium, which is usually due to the poor solubility of chitosan at high pH values.6 It has been reported that oligomers of lower molecular weight than that of chitosan exhibit better biological activities than chitosan. Oligomers of chitosan like chito-oligosaccharide (COSs) could be easily prepared by acidic or by enzymatic partial hydrolysis of chitosan7 Hydrolysis of chitosan can progress by use of acid as hydrochloric acid, nitrous acid, phosphoric acid, hydrofluoric acid, lactic acid, tri-chloroacetic acid, formic acid, and acetic acid have also been studied for their effect of degradation on chitin or chitosan.8 The literature is rife with the reports of a remark ably wide range of biological activities of COSs.8 In the present study, we report the antibacterial activity of chito-oligosaccharide, prepared by acid hydrolysis of chitosan from shrimp shells (Litopenaeus vannamei, Penaeus monodon, Macrobrachium rosenbergii) against three species of bacteria (V. parahaemolyticus, E. coli, P. aeruginosa).
All chemicals used in this study were analytical. Shrimp shells were obtained from a seafood restaurant at Cha-am, Phetchaburi, Thailand. It was confirmed that all shells were from a single species of shrimp. Chito-oligosaccharides were prepared from three shrimp shells that were Pacific white shrimp shells (Litopenaeus vannamei), Giant tiger prawn shells (Penaeus monodon) and Giant freshwater prawn shells (Macrobrachium rosenbergii). The bacteria, namely Vibrio parahaemolyticus, Escherichia coli and Pseudomonas aeruginosa, were used for determination of the antibacterial activity.
Preparation of chitin and chitosan
The shrimp shells were washed under running warm tap water to remove soluble organics, adherent proteins and other impurities. The shells were then collected and boiled in water for 1h to remove the tissue, followed by drying in an oven at 160°C until the shrimp shells dried. At the end, the dried shells were ground into a fine powder using a blender.
Demineralization
Calcium carbonate constitutes the main inorganic component of the shells. To remove the calcium carbonate, only dilute hydrochloric acid was used to prevent hydrolysis of chitin.4 The 1.5M of hydrochloric acid concentrations was used to hydrolyze for 30minute. The ratio of dried shells to acid solution used during the extraction of chitin was 1/15(w/v). The experiments were carried out at room temperature under constant stirring of 150rpm. The decalcified shells were collected on filter paper (No. 1), washed to neutrality with tap water, rinsed with deionizer water, and then oven dried at 80°C overnight.
Deproteinization
Similar experimental conditions were applied for the demineralization of dried shells. The condition of deproteinization was 2.0M sodium hydroxide concentration, the reaction time was 2h and the temperature was 45°C under constant stirring of 150rpm. At the end of this process the material was filtrated, washed and dried, as previously described in the demineralization process.
Decolouration
The chitin residue was mixed with acetone at a solid/solvent ration of 1:10 (w/v) for 10min, filtered, dried for 2h at room temperature, followed by bleaching with 0.315% NaOCl for 5min at the same solid/solvent ration.4 The decoloured chitin was washed and filtered as described previously.
Deacetylation
The conversion of chitin to chitosan involved deacetylation using the process suggested by Kurita et al.,9 The parameters employed (i.e. reaction duration, temperature and concentration of alkaline reagent) were as follows: a suspension of 1g of chitin in 50ml of 50% (w/v) sodium hydroxide, was mixed at fixed temperature (90°C) under constant stirring. After 3h, the solid was filtered, washed with water and 95% (v/v) alcohol until the filtrate was neutral. Then it was oven dried at 80°C overnight.9
Preparation of chito-oligosaccharides
Chitosan (2g) was dissolved in 100ml of hydrochloric acid (37%) until the content became a gelatinous paste and the suspension heated for 30min at 72°C under stirring. The solution was cooled and poured into 95% ethanol. The precipitate was separated by centrifugation, washed three times with 95% ethanol, and then it was oven dried at 80°C overnight.1,10
TLC of chito-oligosaccharides
The chito-oligosaccharides were separated by TLC on silica gel plates (MERCK 60. GF-254) using n-propanol: water: concentrated ammonia 7:2:1 (v:v:v) as solvent. Spots were visualised by charring with 10% H2SO4 in ethanol.1
Antibacterial tests
Antibacterial activities of chito-oligosaccharides (COSs) were examined as the inhibitory effects against the growth of three bacteria: V. parahaemolyticus, E. coli and P. aeruginosa. The 0.5g of sterile COS was added in 100ml of cultured bacteria suspension in a flask and incubated with shaking at 37°C. The inhibitory effect was estimated periodically by measuring turbidity of the cultured medium at 640nm using a spectrophotometer UV-Visible. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of COS required to inhibit completely bacterial growth after incubation for 24h at 37°C. For determination of the minimum inhibitory concentration (MIC) of COS solutions were added to medium for final COS concentrations of 0.5%, 0.05%, 0.005% and 0.001% (w/v). After incubation, the optical density (OD) of cell growth was detected by spectrophotometer UV-Visible at 640nm. The inhibitory activity (%) was calculated by the following equation (Inhibitory activity (%)={(C-T)/C}×100, where C is the numbers of optical density as the control and T is the numbers of optical density obtained from each tested sample).
Chito-oligosaccharides obtained by acid hydrolysis
The images obtained by stereo microscope (10x) were used to observe the external morphology of the chitosan from shrimp shells wastes (Litopenaeus vannamei; A, Penaeus monodon; B and Macrobrachium rosenbergii; C) that showed in the Figure 1. In its crystalline form, chitosan that produce three shrimp shell had difference morphology which chitosan from shrimp shells of Litopenaeus vannamei was thin layer and glittery whereas Macrobrachium rosenbergii was thick layer and opaque. Due to the diversity of sources of chitosan causes it had different properties.11 Chitosan and its oligosaccharides, which were known to possess multiple functional properties, have attracted considerable interest due to their biological activities and potential applications in the food, pharmaceutical, agricultural and environmental industries.12 The acid hydrolysis of chitosan from three shrimp shells (Pacific white shrimp shells; COS1, Giant tiger prawn shells; COS2 and Giant freshwater prawn shells; COS3) were examined by analysis of products using TLC (Figure 2). The results showed that acid hydrolysis used in producing COSs. After acid hydrolysis, the separation profile of the COS1, COS2 and COS3 showed four major factions. Acid hydrolysis (hydrocholoric, nitrous, phosphoric acid, hydrogen fluoride) and oxidative reductive depolymerization (mediated by peroxide,ozone, and persulfate) were important routes for synthesis of COSs.8 The biological activity of COSs was known to depend on their structure.13 Although some reports mention a size-dependent biological activity of COSs, larger oligomers being more potent, most studies do not consider soluble COSs with degrees of polymerization (DPs) higher than 6.1 Therefore, antibacterial activities of different degrees of deactivation (DD) and depolymerization (DP) of COSs against various species of bacterial were measured.
Antibacterial effect
The antibacterial activity of chitosan and chito-oligomers has been recognized and is considered to be one of the most important properties, corresponding directly to their possible biological applications.14,15 Minimum inhibitory concentrations were important to confirm resistance of microorganisms to an antimicrobial agent and also to monitor the activity of new antimicrobial agents. The minimal inhibitory concentration (MIC) was generally regarded as the most basic laboratory measurement of the activity of an antimicrobial agent against an organism. Antibacterial activities of chito-oligosaccharides (COS) were examined as the inhibitory effects against the growth of three bacteria: V. parahaemolyticus, E. coli and P. aeruginosa. The minimal inhibitory concentrations (MIC) of COSs against bacteria (V. parahaemolyticus, E. coli and P. aeruginosa) were 0.5% (w/v). Figure 3 showed effect of COSs on V. parahaemolyticus, E. coli and P. aeruginosa. The result showed that COS1 and COS3 presented a highest inhibitory effect against the growth of V. parahaemolyticus that were 61.75% and 89.40%, respectively whereas COS2 inhibited similarity growth of E. coli and V. parahaemolyticus (34.44% and 36.87%, respectively). However, all COSs exhibited a bactericidal effect on all bacteria tested but E. coli and P. aeruginosa were inhibited lower than 50%. These present suggest that COS3 was prepared from Giant freshwater prawn shells suitable to use as antibacterial agent.
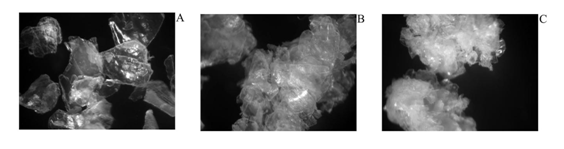
Figure 1 Micrographs of chitosan from shrimp shells wastes (Litopenaeus vannamei; A, Penaeus monodon; B and Macro brachium Rosenberger; C).
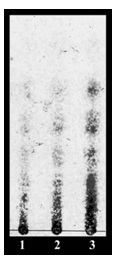
Figure 2 Thin layer chromatography analysis of chito-oligosaccharides (Lane 1, COS 1; Lane 2, COS 2; Lane 3, COS 3). TLC performed on silica gel plate in a solvent system composed of n-propanol, water, ammonia water (7:2:1, v/v/v). The plate was developed by spraying ethanol containing 10% sulfuric acid.
Chito-oligosaccharides (COS) prepared from giant freshwater prawn shells (Macrobrachium rosenbergii; COS3) by acid hydrolysis process were suitable antibacterial agent for inhibited growth of V. parahaemolyticus which inhibitory activity was 89.40%. However, COSs was prepared from different sources that exhibited different antibacterial properties.
The authors would like to thank faculty of animal science and agricultural technology, Silpakorn University for providing necessary facilities to carry out this research.
Author declares that there is no conflict of interest.

©2018 Chimtong. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.