Advances in
eISSN: 2373-6402


Review Article Volume 8 Issue 2
ICAR-Indian Institute of Sugarcane Research, India
Correspondence: Pushpa Singh, ICAR-Indian Institute of Sugarcane Research, Lucknow, 226002, India
Received: November 01, 2017 | Published: March 15, 2018
Citation: Singh P, Rai RK. Tailoring sugarcane for smart canopy architecture. Adv Plants Agric Res. 2018;8(2):142-147. DOI: 10.15406/apar.2018.08.00304
Sugarcane is a crop of great economic importance in sugar and biomass dependent economies because of its exceptionally high productivity and its ability for storage of high concentration of sucrose. It is primarily a crop of tropical and sub-tropical regions of the world. However, the ever increasing demand for sugar and bio fuels have driven the expansion of sugarcane production throughout the world and it is presently being cultivated in nearly 100 countries worldwide with a cultivation area of 23.8million ha and produces 1685 million tonnes cane with an average cane productivity of 71 tha-1. It is the most productive crop with biomass accumulation rates as high as 550 kg ha-1day-1 and provides third highest quantity of plant calories in human diet after rice and wheat. Despite the productivity of sugarcane being highest among crop plants, there exists wide gap in its theoretical yield (470mt ha-1 year-1) and productivity achieved at field (168 mt ha-1year-1) with average cane yield hovering around 65 tonnes ha-1. In India, which is second largest sugarcane producer in the world after Brazil, this productivity lingers around 28 % of the theoretical maximum. The prevalence of short duration crop cultivation practices like- spring and late planting of sugarcane further aggravate the situation. Besides, numerous physiological constraints contributing to low productivity, short duration crop practices mainly succumb to two serious constraints in form short crop growth period and prevalence of high and desiccating temperatures coinciding with the crucial germination and tillering phases. This reduces the crop yields to 30-40 tha-1 and further broadens the gap between theoretical yield and actual yield. Filling the wide yield gaps in sugarcane cropping system is thus a critical target for which realistic measures are required.
Further the amount of land dedicated to sugarcane farming is shrinking every year. And with burgeoning human population, the demand for sugar and it’s by-products are increasing day by day. Though attempts in past decades have led to significant increase in sugarcane productivity, the future demands will have to be addressed through novel approaches for enhancing biomass and sugar production. At present, sugarcane crop in a growth cycle of 12 months;
The physiological barriers in sugarcane can be addressed under the dynamic balance of axillary bud differentiation, above ground and below ground growth for inducing architectural changes in plant parts vis a vis leaves, stems and roots. This is required to be initiated at the germination stage itself. As sugarcane is a vegetative crop, propagated through axillary bud on three budded sets, the delay in germination is required to be addressed through enhancing the heterotrophic to autotrophic transitions. Instead of duration of 45 days, the germination process needs to be reduced to 7 days. This shall render the establishment of higher settlings at an early stage (45 and 90 DAP) unlike under normal conditions where the settling numbers are few due to which the canopy development is poor from this stage itself. Smart canopy development and architecture is an important factor determining yield of many crops as a result of interplant competition for light distribution and absorption, particularly in a dense population.
Higher tiller numbers in a clump in sugarcane with altered architecture will improve the source activity and sink strength and its capacity causing improved growth from 90 DAP itself. Smart canopy functions (e.g. photosynthesis) improve as leaf area index (LAI) increases until LAI reaches approximately7 for sugarcane (Saccharum spp. hybrids), but decrease with further increase in LAI. Correspondingly, the number of stalks, intermodal length and their weights in a clump with higher density will increase. In order to attain higher plant density, sugarcane plants with erect leaves and smart canopy will be of prime importance. Photo synthates for sugarcane yield is produced largely by 7-8 leaves below the apical meristem, but these leaves are largely shaded at high plant density, resulting in reduced cane yield. Settling and stalk growth of sugarcane are associated with light interception during the grand growth phase. These processes are affected by number, position and architecture of leaves. Keeping this relationship in mind, it would be possible to achieve a further increase in cane and sugar yield of Saccharum hybrids if plant architecture could be improved through smart canopy development. Plant architecture depends strongly on morphological and physiological parameters such as leaf area, angle and orientation, photosynthesis and dry matter accumulation as shown below in picture.
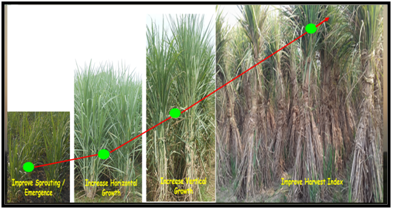
Exploiting Indeterminate Growth Potential–Smart canopy Development
It is difficult or impossible to collect robust field measurements of so many parameters, because of the costs of time and labor. However an opinion on tailoring sugarcane for smart canopy development is listed below.
The approach shall lead to bigger clumpssize with erect leaf architecture, smart canopy delimiting the sink strength and it capacity resulting in improved biomass and sugar contents. Plant growth hormones (usage of Ethrel and GA3) have potentially improved the sett vigour, enhanced its ability to sprout and established uniform and robust settlings in spring planted crop (Figure 1).
The work was conducted at Indian Council of Agricultural Research (ICAR)-Indian Institute of Sugarcane Research, Lucknow, India, located at 26o 56' N, 80o52'E and 111 m above sea level. This falls in Agro-Eco-region 4 (Northern plain and Central Highlands) and Hot Semi-arid Eco-region with Alluvial-derived (N8D2) soils.1 The soil of experimental field was sandy loam (13.3% clay, 24.5% silt and 62.2% sand) of Indo-Gangetic alluvial origin, very deep (>2m), well drained, flat and classified as non-calcareous mixed hypothermic udic ustochrept. The climate of experimental site is semi-arid, sub-tropical with hot dry summers and cold winters. The average monthly minimum and maximum temperature during summer (April to June) range from 18.4oC to 43oC and in winter (November to February) from 7.4oC to 29oC. The average annual rainfall is 1045.5 mm and cumulative open pan evaporation is 1750 mm. nearly 72% of the total rainfall is received through north-west monsoon during July to September. The organic carbon (OC) content of soil was 0.48 % with total nitrogen 0.069 %. The available nitrogen (N), phosphorus (P), potassium (K) were 183.7, 18.7, 192 kg ha-1 in 2012-2013 and 185.6, 18.2, 190 kg ha-1 in 2013-2014.
Sugarcane crops were planted with sugarcane variety CoLk 94184 under autumn, spring and late planted conditions, respectively, at institute farm of Indian Council of Agricultural Research- Indian Institute of Sugarcane Research (ICAR-IISR), Lucknow, India. Both fields with left over wheat stubbles were irrigated and later on prepared with cultivator (once) and harrow (twice). Soil moisture of 16 % was maintained in both fields during planting. Ridges and furrows were laid out at 75 cm spacing with tractor mounted furrow opener. The opened furrows were treated with Chlorpyriphos (20% emulsifiable concentrate) for termites control.
Crop culture and exogenous applications of Ethrel and GA3 at critical growth stages
Sugarcane variety CoLk 94184, was planted under seven treatments. The treatments were T1: Unsoaked (Control), T2: Un soaked+Water application, T3: Unsoaked+GA3 application, T4: Water soaked, T5: Water soaked + GA3 application, T6: Ethrel soaked, T7: Ethrel soaked+GA3 application (Table 1). Prior to planting, sugarcane setts were soaked in Ethrel 100 ppm Ethrel. The setts were left overnight and taken out next morning for planting. They were rinsed in Bavastine (@ 2 g L-1) prior to their planting in the furrows. The foliar application of GA3 was performed at 90, 120 and 150 days after planting (DAP) in T3, T5 and T7. GA3 were dissolved in 0.5 cm3 in ethanol and diluted with distilled water to a concentration of 100 m mol m–3 and applied with knap sac (5 ml per plant) in between 8.00-9.00 AM, while T2 was applied with an equal quantity of distilled water. The concentration of dissolving solvents was too low for any physiological effect on plants. The total quantity of water application and water used in GA3 solution varied with number of plants in every row. Ethrel and Gibberellic acid were purchased from Chemical Drug House (CDH) Bio chemicals, Analytical Reagent (AR) grade, with minimum assay of about 39 % and 99.9 % respectively. The crops were raised with standard agronomic cultivation practices and recommended doses of N, P, and K (150: 80: 80 Kg ha-1). Fertilizers used were urea (46% N), single super-phosphate (6.8% P) and Muriate of Potash (46.2% K). One third of N, P and K were applied as basal dressing in furrows at the time of planting. The remainder N was top-dressed in two equal splits at 45 and 90 DAP. Both the crops received a total of four irrigations and three inter-cultural operations. Application of insecticides was made as per recommendation for the region. The plants in all the treatments were free of pests and diseases during the experiments.
Crop Growth stage |
PGR used |
Concentration(ppm) |
Mode / Time of application |
At planting |
Ethrel |
100 |
Overnight soaking of |
60 DAP |
Ethrel |
100 |
Foliar application 100 |
90 DAP |
GA3 |
35 |
Foliar application of GA3 at specific leaf sites in |
120 DAP |
GA3 |
35 |
Foliar application of GA3 at |
150 DAP |
GA3 |
35 |
Foliar application of GA3 at |
Table 1 Mode/Time of application of PGR at different sugarcane growth stages
Bud sprouting, initial shoot numbers determination and biochemical analysis of cane
Sprouting % was calculated by counting number of sprouted buds out of the total planted buds. The buds with initial shoot protrusion of at least 2 mm in length were considered to be sprouted Rai & others2 Bud moisture, bud dry weight and relative growth rate (RGR) were recorded with 45 buds scooped from 15 setts from respective treatments. The freshly removed buds were washed thoroughly and dried with Whatman No.1 filter paper for recording fresh weight. Bud dry weight was recorded by drying buds in hot air oven, at 102oC for 24 hrs and 80oC for 72 hours, till constant weight. RGR was computed using the formula
ln W2 - ln W1/ t
Where ln W1 is initial bud dry weight and ln W2 is bud dry weight attained after time (t) in days, at 20 and 45 DAP. The numbers of settlings sprouted per plot were counted manually for recording initial plant population.
Freshly sampled bud tissues were chopped and homogenized to prepare 10 % homogenate in a chilled pestle and mortar with chilled distilled water. The homogenate was filtered through four layers of cheese cloth and then centrifuged at 8000 g for 20 min at 4°C. The supernatant obtained after centrifugation was used for estimation of reducing sugars, sucrose and total phenolic contents. Estimation of reducing sugar was done according to method of Nelson3 & Somogyi, 1945. Sucrose was estimated by resorcinol Thio urea method described by Roe & Papadopoulos.4 Protein was estimated by method of Lowry & others5 and acid invertase activity was assayed by method of Hatch & Glasziou.6 Total phenolic contents were estimated by method described by Swain and Hillis.7 Indole acetic acid (IAA) was determined by method of Nagar8 and IAAO activity was assayed by method of Gordon & Weber.9 The ATP ase activity was assayed by method of Fischer & Hodges.10 The phosphorus estimation was done by following the method of Fiske and Subbarow, 1925. NR activity in vivo and SOD activity were assayed by method of Jaworski and others11 & Beauchamp & Fridovich12 respectively.
Determination of leaf characteristics and shoot development
The area per leaf was calculated by method described by Lerch & others.13 The total leaf area of individual stalks was obtained by summation of leaf area on each stalk. Leaf area index (LAI) was calculated by multiplying the mean value of leaf area per stalk by number of stalks present in known area. The growth parameters were individually calculated using formulae of K vet & others.14 Net assimilation rate (NAR)=(W2_ W1) (lnL2_ lnL1)/ [(t2_ t1) (L2 _ L1)], dry matter produced per leaf area and time units (mg cm-2 d-1)-2. With these values and shoot numbers, NAR was calculated on land area basis.
Leaf area ratio (LAR) = L/W, relates leaf area with total stalk dry matter (cm2 g-1).
Leaf area duration (LAD) = L2+L1 (t2 - t1)/2 (cm2 day) 104.
Biomass duration (Z) = W2+W1 (t2 - t1)/2 (g day) 103.
Where W and L are mean values of dry weight and leaf area at a specific time respectively. W1 and W2 represent initial and final mean values of total dry weight of a stalk. L1 and L2 are the initial and final mean values of leaf area belonging to a stalk over the period t2-t1. The growth parameters were calculated at 180 and 270 DAP. The shoot numbers in each plot were counted at 30 days interval till 270 DAP. Stalk length, intermodal length, intermodal girth and intermodal weight were measured with meter scale; vernier calliper and electronic balance in 21 stalks sampled at 180 and 270 DAP.
Determination of total dry matter and juice quality
Stalk weight was recorded by chopping stalk into small pieces. The chopped pieces were dried in an oven at 80oC, until constant weight was achieved. Dry matter content m-2 was quantified from total number of stalks in an area of 16 m2 selected from each plot at 180, and 270 DAP. Cane juice quality was analysed from total number of stalks harvested from an area of 16 m2 at 270 DAP Meade & Chen.15
Statistical analysis
Data was analysed using the statistical product and service solution version 16.0 software (SPSS Inc, Chicago, II). One way analysis of variance with Duncan’s Multiple Range Test (DMRT) as post hoc analysis was used to compare the means Snedecor & Cochran.16 Graphics were generated using Sigma Plot version 10.0 (System software, Inc., Point Richmond, CA). Regression analysis and correlation coefficients were calculated using MS Excel statistical tools to assess the interrelationships between treatment means across the temperature among different parameters.
Their usage led to significant alteration in leaf orientation (Figure 2), cane length (Figure 3) and root architecture (Figure 4). Architectural alterations caused faster heterotrophic to autotrophic transitions at planting stage (February). This induced high initial plant population (45 DAP), which was followed by induction of smart canopy with increased source activity, above and below ground sink development at 60 DAP. The formation of smart canopy was due to development of Electrophiles (leaf angle 73o) against planophiles (leaf angle 45o) in control (Figure 2). Changes in leaf angle enabled added advantage of enhanced CO2 utilization and radiation use efficiency. The GA3 induced leaf orientation formed a smart canopy and improved dry matter partitioning. Further, leaf erectness also reduced the shading effects amongst the leaf present on a stalk, rendering increase in radiation use by lower leaf laminae of the stalk. GA3 induced branched roots with steep angles (30o), threefold increase in root weights and root hair development, sustained the nutrient requirement of increased shoot population. As a result, net assimilation rates [0.65 (cm2 d-1)2], leaf area ratio (16 cm2 g-1) and leaf area duration [55 (cm2 d) 104] enhanced, leading to increase in internodal numbers, length and their weight. At grand growth and harvest stage, a Tmax 5.37 lakhs shoots ha-1 with NMC of 3.01 lakhs ha-1 with Ethrel and GA3 was obtained against Tmax 2.13 lakh shoots ha-1 in control with NMC of 1.32 lakhs shoots ha-1. The application led to significant increase in cane yield of 255 tha-1 (per cane weight 847 g) against a cane yield of 84.69 tha-1 in control (per cane weight 640 g). The large accommodation of stalks in limited ground area with Ethrel and GA3 is explained to be due to the development of smart canopies supported by a robust root system, where each plant occupied merely 331 cm2 ground areas against 800 cm2 in control. The architectural alterations through PGR in sugarcane crop increased cane yield from 70-85 tha-1 to 255tha-1 in spring planted sugarcane crop.17–20

None.
None.

©2018 Singh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.