Advances in
eISSN: 2373-6402


Research Article Volume 8 Issue 1
1Department of Wildlife and Biodiversity Conservation, North Orissa University, Baripada, India
2Department of Botany, North Orissa University, India
Correspondence: RK Mishra, Department of Wildlife and Biodiversity Conservation, North Orissa University, Takatpur, Baripada-757 003, India
Received: February 05, 2016 | Published: January 9, 2018
Citation: Mishra RK, Parhi S, Biswal AK. Diversity of over storey plant communities of tropical forest covers of Balasore district, Odisha, India. Adv Plants Agric Res. 2018;8(1):20-26. DOI: 10.15406/apar.2018.08.00285
The structure and function of a forest ecosystem is maintained by upper storey vegetation layer which principally consist of tree species. The tropical forest covers of Balasore, one of the coastal district of Odisha was analysed for structure, composition and diversity of upper storey vegetation layer (≥30cm circumference at breast height). A total of 94 tree species representing 77 genera and 38 families were recorded in this area. The average number of species per family was nearly equal to 2.5 and per genus was>1.2.The species diversity index and concentration dominance of the forest were 3.68 and 0.055, respectively. The Importance Value Index (IVI) of species ranged from 0.55 to 40.47. The estimated diversity indices indicated heterogeneity of the tropical forest covers of Balasore district in composition, structure and function. Thus rich over storey plant diversity with many of them as rare occurrence in the area supports the need of conservation for future use and sustenance.
Keywords: floristic composition, species diversity, concentration of dominance, IVI, conservation
Vegetation diversity assessment in tropical forests have mostly been concentrated on tree species than other plant life forms, because tree species diversity is an important aspect of forest ecosystem structure and fundamental to tropical forest biodiversity. Tropical forests, the major repository of biodiversity, are undergoing rapid fragmentation and degradation all over the world.1 These covers 7% of the earth’s land surface, but harbours more than half of the world’s species2 and are currently disappearing at an overall rate of 0.8 to 2% per year.3 The declining of tropical forests in different parts of the world is most probably due to the activities of human kind.4 Phytosociology is the study of the characteristics, classification, relationship and distribution of plant communities. It is useful to collect data on the quantitative change of each species studied and how their relationship with other species in the same community. Further, such studies serve as a pre-requisite for investigating the details of primary productivity of tropical ecosystems and can be used for environmental impact assessment studies in future with reference to understand the changes experienced in the past and continuing on into the future.
Most of the developed and developing countries have these basic studies and defined with the help of vegetation maps.5 Presently as the tropical forests in most part of the world are under immense anthropogenic pressure require careful management intervention to maintain their overall biodiversity and sustainability.6 The Indian subcontinent rich in biodiversity and as the 12mega-diversity centres of the world facing problem in disappearance of tropical forests at an alarming rate due to anthropogenic activities and invasion of invasive species.7 During the period 2009 to 2011 net and gross deforestation rate in India was -0.03 and -0.43, respectively.8 In Odisha, forest covers covering about 37.34% of the state’s geographical area and about 7.66% of country’s forest9 having net and gross deforestation rate during 1935 to 2010 was -0.69 and -0.79, respectively.8 Balasore, one of the coastal districts of Odisha located at 20° 48’to 21° 59’ north latitude and 86° 16’ to 87° 29’ east longitude having a total forest area of 351 sq.km consisting of 23 sq.km of dense forest, 126 sq. km of moderate dense forest and 202sq. km of open forest.10 A study mentioned that the gross deforestation rate of the district was found 0.69 with in a period of 31years from 1973 to 2004.8 As per the State of Forest Report, Govt. of India from 2003 to 2007 the comparative account of decrease in close forest cover including very dense and moderate dense forest of the district was 1.5sq. km per year. Thus accurate quantification of vegetation especially over storey vegetation layer is a prerequisite to provide information in formulating various action plans or management plans for their restoration. In these contexts vegetation analysis pertaining to Phytosociology and community structure of over storey vegetation layer in different forest covers of Odisha was made by various workers.11–13 However, it remains as a neglected area with no such studies was made for the tropical forest covers of Balasore. Keeping paucity on quantitative information on over storey plant composition, diversity, and community characteristics the present study was designed to assess the species diversity, richness, abundance and distribution of over storey vegetation layer existing in the study area.
Study area
The Phytosociological study of upper storey vegetation layer (tree) was carried out in Balasore district, Odisha. The district lies between 20° 48’ to 21° 59’ north latitude and 86° 16’ to 87° 29’ east longitude (Figure 1). The district is surrounded by Medinipur district of West Bengal in its northern side, Bay of Bengal in its east, Bhadrak district in its south and Mayurbhanj and Keonjhar districts on its western side. The climate of the district is mostly hot and humid. Annual mean temperature and precipitation of Balasore is 32 °C and the average rain fall is 1583mm.14 Relative humidity is generally high throughout the year and varying in the range of 50% to 90%. The district total geographical area is 3806 sq km. Nearly 9.22% of the geographical area of the district is covered by forest which plays an important role in the economy of the district.
Phytosociological analysis
Upper storey vegetation layer of tropical forest covers of the district was analysed by quadrat method. A total of 42 quadrats of 20 m x 20m size were laid down at different aspects. The size and number of quadrats laid down during survey was ascertained as per species area curve15 and running mean method.16 All the tree species in quadrats were enumerated and identified following Saxena & Brahmam.17 All the trees occurring inside the quadrat were recorded and measured for girth at breast height level (1.37m) from the ground. Quantitative data collected during field survey were subjected to analyze various phytosociological parameters following standard methods of calculations and formulae.15,16,18-20 The frequency values obtained were grouped in frequency classes to study the homogeneous/heterogeneous nature of vegetation.21 To assess the overall impact of a species Importance Value Index (IVI) was determined by adding relative frequency, relative density and relative basal area as per Cottam & Curtis.18
Relative Frequency (%): Relative frequency is the degree of dispersion of individual species in an area in relation to the number of all species occurred. It was determined following the equation as
Relative Density (%): Relative Density is the numerical strength of a species in respect to the total number of individual of all the species. It was calculated following the equation as:
Relative Dominance (%): Dominance is the parameter which is determined by the value of basal area. For the comparative analysis relative dominance is determined. It is the coverage value of a species with respect to the sum of coverage of the rest of the species in the area.It was calculated as:
Importance Value Index: Importance Value Index is used to determine the overall impact of each species in the community structure. It was calculated by the addition of the percentage values of the relative frequency, relative density and relative dominance (Relative basal area).
Species diversity of upper storey vegetation layer was determined with the Shannon-Wiener diversity index.22 Concentration of Dominance (CD) was calculated to evaluate the level of dominance of a species within a community and was expressed by Simpson’s index.23
Shannon-Wiener index (Shannon & Wiener, 1963): It is a measure of the average degree of ‘uncertainty’ in predicting to what species an individual chosen at random from a collection of S species and N individuals. It was estimated by using formula:
Where, ni: Number of individuals belonging to the species
N: Total number of individuals in the sample
Dominance index (Simpson, 1949): It is a measure of dominance since it weighted towards the abundances of commonest species. It was estimated by using formula:
Where “ni” and “N” are same to Shannon-Wiener index.
Floristic composition
A total of 94 species belonging to 77 genera and 38 families were recorded from the study area. A majority of the families were represented by only one or two species (Table 1). The most common families were Euphorbiaceae (10 species) and Rubiaceae (8 species) followed by Combretaceae (5 species), Caesalpiniaceae = Ebenaceae = Anacardiaceae = Apocynaceae = Verbenaceae = Oleaceae (4 species each), Rutaceae = Barringtoniaceae (3 species each), etc. The number of species per genus was more than 1.2 and that per family was nearly equal to 2.5. The species richness of 94 species over 16.8 ha area of the district reflects a moderate level of diversity in forests of Eastern Ghats (Table 1). The results of the study compared well to other inventories conducted in tropical forests both in India and elsewhere. Murphy and Lugo24 emphasized that average tree species richness of a dry tropical forest community ranges from 35 to 90. Condit25 recorded 63 species from 50 ha plot at Mudumalai Forest Reserve, India to 996 species in 52 ha plot at Lambir, Malaysia. In a recent assessment of species richness in southern Eastern Ghats, Pragasan and Parthasarathy26 recorded 272 species in 60 ha sampled area.
Distribution pattern of species
Of the 94 tree species recoded in the study area most of the species exhibited contiguous distribution (70 species). Only few species exhibited random (17 species) and regular (7 species) distribution. Contiguous distribution was more pronounced by Chionanthus macrophylla, Premna calycina, Soymida febrifuge, Tectona grandis, Ixora pavetta, Murraya paniculata, Strychnos nox-vomica, etc. Species showed regular distribution were Anogeissus latifolia, Macaranga peltata, Holarrhaena pubescens, Careya arborea, Fragerlindia fasciculate, Syzygium cumini and Casearia graveolens (Table 1).
Raunkiaer’s frequency class distribution
The over storey species had a wide range of occurrence in the study site ranging in frequency from 4.76 to 66.67 % and most of the species occurred only twice (Table 1). The distribution of the species into Raunkiaer’s frequency classes showed that most of the species encountered were in class “A” followed by class “B” and equal in “C” and “D” (Figure 2). This indicates that most of the species had low frequency as would be expected in typical species-abundance distribution in tropical forests.27 Raunkiaer21 frequency distribution holds good for this forest cover also. However this forest had only four classes instead of five as described in the law. This was due to its higher heterogeneous nature and deviation from the normal frequency distribution as described by Raunkiaer.21
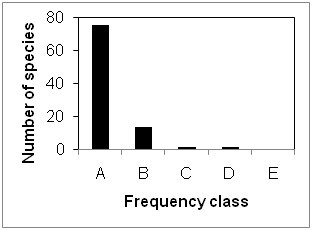
Figure 2 Raunkiaer’s frequency class distribution of over storey vegetation layer of the study area.
Stand structure
Structural parameters like density (plants/ha), basal area (m2/ha) and frequency (%) of upper-storey vegetation layer of the study area is presented in Table 1. Species wise density of individuals having ≥30cm girth ranged from 2 to 134 plants/ha and the total density of 729 plants/ha. Maximum density was recorded for Shorea robusta (134) followed by Terminalia alata (70), Croton roxburghii (25), Boswellia serrata (25), Buchnania lanzan (22.62), Madhuca indica (21.43) and Xantolish tomentosa (20.24). The minimum density of less than equal to two was observed for many species like Aporusa octandra, Spondias pinnata, Miliusa velutina, Bauhinia purpurea, Sterospermum colais, Litsia monopetala, Vitex penducularis, Syzygium cerasoides, etc. Rest of the species showed intermediate range of density per hectare.
Name of the Species |
Family |
Density (Plants/ha) |
Frequency (%) |
Basal Area (m2/ha) |
A/F |
IVI |
Species Diversity(H') |
Concentration of Dominance (Cd) |
Acacia leucophloea (Roxb.) Willd |
Mimosaceae |
2.38 |
9.52 |
0.009 |
0.049 |
1.13 |
0.019 |
0.000011 |
Acacia pennata ( L.)Willd. |
Mimosaceae |
1.19 |
4.76 |
0.144 |
0.11 |
1.05 |
0.01 |
0.00003 |
Actinodaphne angustifolia (Bl.) Nees |
Lauraceae |
4.76 |
9.52 |
0.1 |
0.1 |
1.81 |
0.033 |
0.00004 |
Aegle marmelos (L.) Corr. |
Rutaceae |
2.38 |
9.52 |
0.096 |
0.049 |
1.43 |
0.019 |
0.000011 |
Alangium salvifolium (L.f.)Wang. |
Alangiaceae |
1.19 |
4.76 |
0.053 |
0.11 |
0.74 |
0.01 |
0.000003 |
Alstonia scholaris (L.) R. Br. |
Apocynaceae |
2.38 |
4.76 |
0.2 |
0.2 |
1.41 |
0.019 |
0.000011 |
Anogeissus latifolia (Roxb.ex.Dc.)Wall.exGuill. &Perr. |
Combretaceae |
9.52 |
33.33 |
0.233 |
0.02 |
4.82 |
0.057 |
0.0002 |
Anthocephalus chinensis (Lam.)A. Rich. ex Walp. |
Rubiaceae |
4.76 |
9.52 |
0.1 |
0.1 |
1.91 |
0.033 |
0.00004 |
Antidesma ghasembilla Gaertn. |
Euphorbiaceae |
1.19 |
4.76 |
0.03 |
0.1 |
0.66 |
0.01 |
0.000003 |
Aporusa octandra ( Buch.-Ham.exD.Don) |
Euphorbiaceae |
1.19 |
4.76 |
0.007 |
0.1 |
0.57 |
0.011 |
0.000003 |
Barringtonia acutangula (L.) Gaertn. |
Barringtoniaceae |
1.19 |
4.76 |
0.13 |
0.1 |
1.02 |
0.011 |
0.000003 |
Bauhinia malabarica Roxb. |
Caesalpiniaceae |
1.19 |
4.76 |
0.174 |
0.1 |
1.16 |
0.011 |
0.000003 |
Bauhinia purpurea L. |
Caesalpiniaceae |
1.19 |
4.76 |
0.019 |
0.1 |
0.62 |
0.011 |
0.000003 |
Bauhinia semla Wunderl |
Caesalpiniaceae |
2.38 |
4.76 |
0.203 |
0.2 |
1.42 |
0.018 |
0.000011 |
Bombax ceiba L. |
Bombacaceae |
9.52 |
19.05 |
0.631 |
0.049 |
5.05 |
0.057 |
0.000171 |
Boswellia serrata Roxb.ex Colebr. |
Burseracee |
25 |
33.33 |
1.16 |
0.04 |
10.18 |
0.116 |
0.00118 |
Bridelia airy-shawii P.T.Li |
Euphorbiaceae |
3.571 |
14.29 |
0.23 |
0.03 |
2.45 |
0.026 |
0.000024 |
Buchanania lanzan Speng. |
Anacardiaceae |
22.62 |
28.57 |
0.375 |
0.049 |
6.73 |
0.107 |
0.000964 |
Canthium dicoccum (Gaertn.)Teijsm & Binnend. |
Rubiaceae |
3.57 |
9.52 |
0.041 |
0.07 |
1.4 |
0.026 |
0.000024 |
Careya arborea Roxb. |
Barringtoniaceae |
7.14 |
23.81 |
0.07 |
0.025 |
3.15 |
0.045 |
0.0001 |
Casearia graveolens Dalz |
Flacourtiaceae |
4.76 |
19.05 |
0.019 |
0.02 |
2.26 |
0.033 |
0.000043 |
Cassia fistula L. |
Caesalpiniaceae |
7.14 |
19.05 |
0.059 |
0.08 |
2.73 |
0.045 |
0.00001 |
Chionanthus macrophylla (Wall.ExG.Don)Bl. |
Oleaceae |
5.95 |
4.76 |
0.076 |
0.5 |
1.47 |
0.039 |
0.00007 |
Chionanthus mala- elengi (Dennst.) Green |
Oleaceae |
8.33 |
14.29 |
0.264 |
0.08 |
3.22 |
0.051 |
0.000131 |
Cleistanthus collinus (Roxb.) Benth. ex. Hook.f. |
Euphorbiaceae |
8.33 |
14.29 |
0.131 |
0.08 |
2.76 |
0.051 |
0.000131 |
Combretum roxburghii Spreng. |
Combretaceae |
1.19 |
4.76 |
0.001 |
0.11 |
0.56 |
0.01 |
0.000003 |
Croton roxburghii Balak |
Euphorbiaceae |
25 |
38.09 |
0.493 |
0.03 |
8.23 |
0.116 |
0.001177 |
Cycas circinalis L. |
Cycadaceae |
1.19 |
4.76 |
0.01 |
0.1 |
0.59 |
0.01 |
0.000003 |
Dalbergia lanceolaria L.f. |
Moraceae |
2.38 |
4.76 |
0.29 |
0.2 |
1.73 |
0.018 |
0.000011 |
Dalbergia latifolia Roxb. |
Fabaceae |
2.38 |
9.52 |
0.23 |
0.049 |
1.9 |
0.019 |
0.000011 |
Dalbergia volubilis Roxb. |
Fabaceae |
1.19 |
4.76 |
0.002 |
0.1 |
0.56 |
0.01 |
0.000003 |
Dillenia pentagyna Roxb. |
Dilleniaceae |
11.91 |
23.81 |
1.299 |
0.04 |
8.1 |
0.067 |
0.0003 |
Diospyros sylvatica Roxb. |
Ebenaceae |
4.76 |
14.29 |
0.037 |
0.049 |
1.94 |
0.033 |
0.000043 |
Diospyros malabarica (Desr.) Kostel |
Ebenaceae |
5.95 |
9.52 |
2.215 |
0.13 |
9.33 |
0.039 |
0.00007 |
Diospyros melanoxylon Roxb. |
Ebenaceae |
17.86 |
23.81 |
0.116 |
0.06 |
4.78 |
0.09 |
0.000601 |
Diospyros montana Roxb. |
Ebenaceae |
7.14 |
14.29 |
0.122 |
0.07 |
2.56 |
0.045 |
0.0001 |
Erhetia laevis Roxb. |
Ehretiaceae |
1.19 |
4.761 |
0.001 |
0.1 |
0.56 |
0.011 |
0.000003 |
Fagerlindia fasciculate (Roxb.)Tirveng. |
Rubiaceae |
9.52 |
28.57 |
0.2 |
0.02 |
4.33 |
0.057 |
0.000171 |
Garuga pinnata Roxb. |
Burseracee |
1.19 |
4.76 |
0.014 |
0.1 |
0.59 |
0.01 |
0.000003 |
Glochidion lanceolarium (Roxb.) Dalz |
Euphorbiaceae |
16.66 |
19.05 |
0.196 |
0.09 |
4.51 |
0.086 |
0.000523 |
Grewia tilifolia Vahl. |
Tiliaceae |
1.19 |
4.76 |
0.011 |
0.1 |
0.59 |
0.01 |
0.000003 |
Haldinia cordifolia (Roxb.)Ridsd. |
Rubiaceae |
8.33 |
19.05 |
0.468 |
0.04 |
4.32 |
0.051 |
0.000131 |
Heterophragma roxburghii L. |
Bignoniaceae |
1.19 |
4.76 |
0.007 |
0.1 |
0.57 |
0.01 |
0.000003 |
Holarrhena pubescens (Buch-Ham.)Wall.ex G Don |
Apocynaceae |
19.05 |
57.14 |
0.285 |
0.01 |
8.23 |
0.095 |
0.000683 |
Holoptelia integrifolia (Roxb.)Planch. |
Ulmaceae |
1.19 |
4.76 |
0.016 |
0.1 |
0.6 |
0.01 |
0.000003 |
Ixora pavetta Andr. |
Rubiaceae |
2.38 |
4.76 |
0.011 |
0.21 |
0.75 |
0.019 |
0.000011 |
Ixora undulate Roxb. |
Rubiaceae |
2.38 |
9.52 |
0.048 |
0.049 |
1.26 |
0.019 |
0.000011 |
Kydia calycina Roxb. |
Malvaceae |
2.38 |
9.52 |
0.261 |
0.049 |
2.01 |
0.019 |
0.000011 |
Lagerstroemia paviflora Roxb. |
Lythraceae |
3.57 |
9.52 |
0.037 |
0.07 |
1.39 |
0.026 |
0.000024 |
Lannea coromandelica (Houtt.)Nerr. |
Anacardiaceae |
7.14 |
14.29 |
0.124 |
0.07 |
2.57 |
0.045 |
0.0001 |
Leea asiatica (L.) Ridsdale |
Vitaceae |
1.19 |
4.76 |
0.008 |
0.1 |
0.58 |
0.01 |
0.000003 |
Ligustrum gamblei Ramam. |
Oleaceae |
1.19 |
4.76 |
0.01 |
0.1 |
0.6 |
0.01 |
0.000003 |
Limonia crenulata Roxb. |
Rutaceae |
1.19 |
4.76 |
0.036 |
0.1 |
0.68 |
0.01 |
0.000003 |
Litsea glutinosa (Lour) Robins. |
Lauraceae |
1.19 |
4.7619 |
0.025 |
0.1 |
0.64 |
0.011 |
0.000003 |
Litsea monopetala (Roxb.) Pers. |
Lauraceae |
1.19 |
4.76 |
0.024 |
0.1 |
0.63 |
0.011 |
0.000003 |
Macaranga peltata (Roxb.)Muell.-Arg. |
Euphorbiaceae |
9.52 |
33.33 |
0.062 |
0.02 |
4.22 |
0.057 |
0.000171 |
Madhuca indica Gmel. |
Sapotaceae |
21.43 |
14.29 |
0.232 |
0.2 |
4.91 |
0.104 |
0.000865 |
Mallatous philippensis (Lam.) Muell.-Arg. |
Euphorbiaceae |
1.19 |
4.76 |
0.005 |
0.1 |
0.57 |
0.011 |
0.000003 |
Mangifera indica L. |
Anacardiaceae |
8.33 |
9.52 |
5.451 |
0.18 |
20.98 |
0.051 |
0.000131 |
Melia azadirachta L. |
Meliaceae |
1.19 |
4.76 |
0.045 |
0.1 |
0.71 |
0.01 |
0.000003 |
Meyna spinosa Roxb.ex Link. |
Rubiaceae |
1.19 |
4.76 |
0.011 |
0.1 |
0.588 |
0.011 |
0.000003 |
Miliusa velutina (Dunal) Hook.f.& Thoms. |
Annonaceae |
1.19 |
4.76 |
0.001 |
0.1 |
0.54 |
0.011 |
0.000003 |
Morinda pubescens Sm. |
Rubiaceae |
1.19 |
4.76 |
0.002 |
0.1 |
0.56 |
0.011 |
0.000003 |
Murraya paniculata (L.)Jack. |
Rutaceae |
2.38 |
4.76 |
0.009 |
0.21 |
0.74 |
0.019 |
0.000011 |
Nyctanthes arbor-tristis L. |
Oleaceae |
1.19 |
4.76 |
0.018 |
0.1 |
0.61 |
0.011 |
0.000003 |
Ochna squarrosa L. |
Ochnaceae |
1.19 |
4.76 |
0.006 |
0.1 |
0.57 |
0.01 |
0.000003 |
Oroxylum indicum (L.) Vent |
Bignoniaceae |
3.57 |
14.29 |
0.086 |
0.03 |
1.95 |
0.026 |
0.000024 |
Phyllanthus emblica L. |
Euphorbiaceae |
3.57 |
9.52 |
0.03 |
0.07 |
1.36 |
0.026 |
0.000024 |
Polyalthia suberosa L. |
Annonaceae |
1.19 |
4.76 |
0.021 |
0.1 |
0.62 |
0.01 |
0.000003 |
Pongamia pinnata (L.)Pierre |
Fabaceae |
19.05 |
19.05 |
1.721 |
0.1 |
10.17 |
0.095 |
0.000683 |
Premna calycina Haines |
Verbenaceae |
5.95 |
4.76 |
0.174 |
0.5 |
1.81 |
0.039 |
0.00007 |
Pterocarpus marsupium Roxb. |
Fabaceae |
13.09 |
23.81 |
0.231 |
0.04 |
4.53 |
0.072 |
0.000323 |
Pterospermum acerifolium (L.)Willd |
Sterculiaceae |
1.19 |
4.76 |
0.041 |
0.1 |
0.69 |
0.01 |
0.000003 |
Schleichera oleosa (Lour.) Oken |
Sapindaceae |
14.29 |
28.57 |
0.31 |
0.03 |
5.35 |
0.077 |
0.000384 |
Shorea robusta Gaertn.f |
Dipterocarpaceae |
134.523 |
66.66 |
4.753 |
0.06 |
40.47 |
0.312 |
0.034092 |
Soymida febrifuge (Roxb.)A. Juss. |
Meliaceae |
3.57 |
4.76 |
0.04 |
0.3 |
1.03 |
0.026 |
0.000024 |
Spondias pinnata (L.f.)Kurz |
Anacardiaceae |
1.19 |
4.76 |
0.001 |
0.1 |
0.57 |
0.01 |
0.000003 |
Sterospermum colais (Buch.-Ham.ex Dillw.) Mabberley |
Bignoniaceae |
1.19 |
4.76 |
0.001 |
0.1 |
0.59 |
0.011 |
0.000003 |
Strychnos nox-vomica L. |
Strychnaceae |
2.38 |
4.76 |
0.041 |
0.21 |
0.86 |
0.019 |
0.000011 |
Syzygium cumini (L.)Skeels |
Myrtaceae |
13.09 |
42.86 |
0.491 |
0.01 |
6.97 |
0.072 |
0.000323 |
Syzygium cerasoides (Roxb.) Chatt. & Kanjilal f. |
Myrtaceae |
1.19 |
4.76 |
0.144 |
0.1 |
1.06 |
0.01 |
0.000003 |
Tectona grandis L.f. |
Verbenaceae |
3.57 |
4.76 |
0.028 |
0.3 |
0.97 |
0.026 |
0.000024 |
Terminalia alata Heyne.ex Roth |
Combretaceae |
70.24 |
61.91 |
1.496 |
0.03 |
19.87 |
0.225 |
0.009294 |
Terminalia bellirica (Gaertn.) Roxb. |
Combretaceae |
10.71 |
23.81 |
0.37 |
0.04 |
4.71 |
0.062 |
0.000216 |
Terminalia chebula Retz. |
Combretaceae |
3.57 |
9.52 |
0.063 |
0.07 |
1.48 |
0.026 |
0.000024 |
Trema orientalis (L.) Bl. |
Ulmaceae |
2.38 |
4.76 |
0.04 |
0.2 |
0.88 |
0.019 |
0.000011 |
Trewia nudiflora L. |
Euphorbiaceae |
4.76 |
9.52 |
0.245 |
0.11 |
2.28 |
0.033 |
0.00004 |
Vitex peduncularis Wall.ex Schauer |
Lamiaceae |
1.19 |
4.76 |
0.021 |
0.1 |
0.62 |
0.01 |
0.000003 |
Vitex pinnata L. |
Verbenaceae |
3.57 |
9.52 |
0.159 |
0.07 |
1.82 |
0.026 |
0.000024 |
Vitex trifolia L. |
Verbenaceae |
1.19 |
4.76 |
0.252 |
0.1 |
1.43 |
0.011 |
0.000003 |
Wendlandia tinctoria (Roxb.) DC |
Rubiaceae |
7.14 |
14.29 |
0.122 |
0.07 |
2.56 |
0.045 |
0.0001 |
Wrightia arborea (Dennst.)Mabb. |
Apocynaceae |
9.52 |
14.29 |
0.119 |
0.09 |
2.88 |
0.056 |
0.000171 |
Wrightia tinctoria (Roxb.) R.Br. |
Apocynaceae |
14.29 |
23.81 |
0.113 |
0.049 |
4.28 |
0.077 |
0.000384 |
Xantolis tomentosa (Roxb.)Rafin. |
Sapotaceae |
20.24 |
28.57 |
0.347 |
0.049 |
6.3 |
0.099 |
0.000772 |
728.474 |
28.453 |
300 |
3.681 |
0.055475 |
Table 1 Species composition, frequency (%), density (Plants/ ha), basal area (m2/ha), A/F ratio, Importance Value Index (IVI), Species diversity (H') and concentration of dominance (Cd) of over storey vegetation layer in tropical forests of Balasore district
The tree density of 729 individuals/ha recorded in the present investigation is lower as compared to densities reported from Saddle Peak of North Andaman Islands and Great Andaman Groups (946-1137 trees/ha,)28 and well comparable to tropical forests of Kalkad, Western Ghats (575-855 trees/ha,)29, Similipal, Odisha,30,31 Brazil (420-777 trees/ha,)32, seasonally deciduous forest of Central Brazil (734 trees/ha,)33, Semi deciduous forest of Piracicaba, Brazil (842 trees/ha,)34 and Costa Rica (617 trees/ha,).35 Basal area of upper storey vegetation in the study area ranged from 0.001-5.451m2/ha, the highest for Mangifera indica and lowest for many species like Ethetia laevis, Sterospermum colais, Spondias pinnata and Miliusa velutina (Table 1). Basal areas for some important timber species such as Shorea robusta, Tectona grandis and Dalbergia lanceolaria were 4.753, 0.028 and 0.290 m2/ha, respectively. The overall basal area estimated was 28.453m2/ha. Mangifera indica contributed maximum of 19% to the total basal area followed by Shorea robusta (16.61%), Diospyros malabarica (7.72%) and Pongamia pinnata (6%). The total contribution that resulted from this associated combination of Mangifera-Shorea-Diospyros-Pongamia was 50%. The overall basal area estimated of upper storey vegetation layer is well within the reported range of various Indian tropical forests36 and lower than the value reported from Monteverde of Costa Rica (62 m2/ha,).37 High basal area is a characteristic feature of mature forest stand and serves as a reflection of high performance of the trees. It may also presuppose the development of an extensive root system used efficient nutrient absorption, growth suppressing of subordinate plants as they intercept much of the solar radiation that might otherwise reach the forest floor.
Ecological importance of species
Importance Value Index (IVI) is the measurement of relative contribution of a species to the entire community and suggesting the ability of a species to establish over an array of habitats. However, there is no single perfect way of assessing the relative contribution of a species. The abundance of a species can be represented by several measures such as relative density, relative frequency and Importance Value Index (IVI). Though frequency and density values are suitable for herbs and shrubs,39 IVI is an important information for tree species. On the basis of IVI, Shorea robusta was found as the dominant species having IVI of 40.47 followed by Mangifera indica (20.98), Terminalia alata (19.87), Boswellia serrata (10.18), Pongamia pinnata (10.17), etc. Miliusa velutina had IVI of 0.054 was considered as the rare species of the study area (Table 1). All other tree species showed intermediate range of IVI. High IVI values exhibited by those species clearly indicate the ecological importance of corresponding species. Such measurement of over storey vegetation layer also helps in understanding the ecological significance of a species in its community/habitat. Higher is the IVI more ecological significance of the species in a particular ecosystem.27 Furthermore, information of IVI would of prime importance in deciding the management options for specific host population of native wildlife that is facing the danger of local extinction due to heavy human pressure surrounding this forest cover.
Diversity measures
Species diversity and concentration of dominance of upper-storey vegetation layer of the study area is given in Table 1. Measurement of biodiversity of specific area (local scale) on the basis of species richness does not provide a complete understanding about the individuals of the species in an ecosystem as it suffers from the lack of evenness or equitability. Shannon Wiener’s index of diversity is one of the popular measures of species diversity. It ranged from 0.01 to 0.312 across the study area with a total diversity value of 3.68. Maximum species diversity of 0.312 was experienced by Shorea robusta while the minimum of 0.01 was experienced by many species of the study area indicating that over storey vegetation layer of Balasore was highly diverse. The species diversity is generally higher for tropical forests, which is reported as 5.06 and 5.40 for young and old stand, respectively.39 For Indian forests the diversity index ranges between 0.83- 4.1.41 Higher species diversity index in tropical forests as reported by Knight40 in comparison to the present investigation may be due to differences in the area sampled and lack of uniform plot dimensions. In contrast to species diversity the concentration of dominance of such vegetation layer ranged from 0.000003 to 0.03. Maximum value was experienced by Shorea robusta and minimum by many species (Table 1). The range of concentration of dominance estimated for over-storey vegetation layer of the district implies that most of the species are equitably distributed while very few species showed the degree of dominance.42 The range of concentration of dominance estimated of the study area is less than those recorded in Nelliampathy (0.085;)43 and tropical dry deciduous forests of Western India (0.08- 0.16;)44 and indicates the absence of single species dominance.
Tropical forest covers of Balasore district supports a diverse plant community. The rich plant diversity is worthy for its conservation to check it from further reduction in species richness, rapid deforestation and forest fragmentation. The rare species of the area identified based on IVI must need proper attention to determine their conservation status and key functions. Further research on mapping of such species with respect to their concentrated distribution in some pockets of the study area and study of their key ecological and structural functions would help to identify locations for conservation actions.
The authors are thankful to the Divisional Forest Officer (DFO), Balasore Wildlife Division and their staff for their help rendered during the study period to carry out the research work.
The author declares no conflict of interest.

©2018 Mishra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.