Advances in
eISSN: 2373-6402


Research Article Volume 7 Issue 5
Department of Biotechnology, Delhi Technological University, India
Correspondence: Navneeta Bharadvaja, Plant Biotechnology Laboratory, Department of Biotechnology, Delhi Technological University, New Delhi-42, India
Received: September 11, 2017 | Published: October 26, 2017
Citation: Roy A, Bharadvaja N. Establishment of the shoot and callus culture of an important medicinal plant Plumbago zeylanica. Adv Plants Agric Res. 2017;7(5):407-409. DOI: 10.15406/apar.2017.07.00274
Plumbago zeylanica is an important medicinal plant which belongs to the Plumbaginaceae family. It possesses antibacterial, hepato protective, and central nervous system stimulatory activity, anti-fungal, anti-inflammatory, anti-hyperglycemic, anti-cancer and anti-atherosclerotic activity. The objective of this study was to assess the effect two cytokinins (BAP and Kinetin) on shoot growth and two auxin on callus induction (2, 4-D and NAA). Highest multiple shoots (4.33±0.63) and length of shoot (5.01±0.53) were induced from nodal explants on modified MS medium supplemented with the 1.0mg/L 6-benzyadenine and 3%. Among the two auxin used for the investigation leave explants showed highest callus induction in MS media supplement with 1.0mg/l 2, 4-D.
Medicinal plants are one of the important sources of natural products, which include essential oils, fragrances, pigments, feedstock, and pharmaceuticals. They are of great interest to the researches in biotechnology field as most of the pharmaceutical industries depend on them Chand et al.1 Plumbago zeylanica is an important medicinal plant which belongs to the Plumbaginaceae family. It is commonly known as Chitrak and Lead word. The plants consists of various bioactive compounds like alkaloids, naphtha quinones, flavonoids, glycoside, steroids, Saponin, phenolic compounds, triterpenoids, coumarins, tannins, carbohydrate, fixed oils, fats, and proteins are present in different plant parts which has been reported to show antibacterial Uma Devi et al.,2 anti-plasmodial, hepato protective, central nervous system stimulatory activity, anti-fungal, anti-inflammatory, anti-hyperglycemic, anti-atherosclerotic activity Kumar et al.3 anti-tumor, anti-cancer Roy et al.4 etc. Propagation of this plant through seed is not reliable due to its poor germination under natural conditions. Pharmaceutical companies procured the plant material from wild and which leads to the depletion of this plant rapidly. This raises the concern about extinction of this. Due to the increasing need of this plant, an alternative technology to the conventional method in improvement of crop is to utilize the biotechnological approaches. In-vitro propagation is currently used to a large number of medicinally important plants for the multiplication and conservation. Earlier reports suggest that this plant can be multiplied by in-vitro propagation Rout, Wei et al.,5–7 Looking at the importance of Plumbago zeylanica, in this study an effort has been done to investigate the role of different cytokinin and auxin elicitors on the growth of the plant. Different types of cytokinins and auxin provide shoot multiplication and callus induction. The present study describes the optimum condition for shoot multiplication and establishment of callus culture from nodal and leaf explants of Plumbago zeylanica.
Plant material
In-vitro grown Plumbago zeylanica accession number- 524441 was collected from the National Bureau of Plant Genetic Resources (NBPGR). This accession was further maintained in MS (Murashige and Skoog medium) media containing 0.2 mg/l BAP in Plant Biotechnology Laboratory of Delhi Technological University.
Effect of different cytokinin concentration on shoot induction
Nodal explants were cultured in modified MS (NaNO3 instead of NH4NO3) media containing BAP (benzyl amino purine) (1.0 mg/l, 1.5 mg/l and 2.0 mg/l) and Kinetin (1.0 mg/l, 1.5 mg/l and 2.0 mg/l) for shoot multiplication. pH of the medium was adjusted to 5.8±0.2 by using 1N NaOH or 1N HCL before autoclaving at 121°C for 20 min. 0.8% (w/v) agar was used as a gelling agent. The culture was incubated at 25±2°C with 16h/8h photoperiod. Visual observation was made weekly and data were recorded. After eight weeks shoot number and shoot length were recorded.
Effect of different auxin concentration on callus induction
For induction of callus, leaves from the in-vitro grown plant were excised (0.5 cm) and cultured on modified MS (NaNO3 instead of NH4NO3) media supplemented with 2,4-D(2,4-dichlorophenoxyacetic acid) (1.0 mg/l), 2,4-D (1.0 mg/l) + BAP (0.5 mg/l), NAA (Naphthalene acetic acid) (1 mg/l) and NAA (1.0 mg/l) + BAP (0.5 mg/l).
Statistical analysis
Experiment was repeated thrice and each treatment had five replicates. The number of cultures per replicate varied for the experiments. Observations were recorded as the number of shoots per explants and length of shoot per nodal explant. The data were analyzed using one-way analysis of variance (ANOVA) (p<0.05).
Effect of different cytokinin concentration on shoot induction
In the present two different cytokinins were used i.e. BAP and Kinetin, BAP showed more effective proliferation of shoots than kinetin (Table 1), (Figure 1) express the effect of different concentration (1 mg/l, 1.5mg/l and 2mg/l) of cytokinins on the shoot number and shoot length. Proliferation of shoots was observed in all the culture media after two weeks of incubation. There was very less proliferation observed in MS media without any growth hormone. At high concentration of both the cytokinin, shoot proliferation rate declined. Highest shoot proliferation and length was achieved in the modified MS (NaNO3 instead of NH4NO3) supplement with the 1.0 mg/l BAP. In the present study modified MS media was used for the first time to observe the effect on the growth of P. zeylanica and also the entire study was done on the P. zeylanica accession which was genetically identified and supplied by NBPGR.
Media |
Number of Shoot |
Length of Shoot |
MS+1 mg/l BAP |
4.33±0.63 |
5.01±0.53 |
MS+1.5mg/l BAP |
2.6 ±0.69 |
3.48±0.44 |
MS+2mg/l BAP |
1.8±0.63 |
1.47±0.43 |
MS+1 mg/l Kn |
2.5±0.53 |
2.62±0.46 |
MS+1.5mg/l Kn |
1.0±0.48 |
1.08±0.34 |
MS+2mg/l Kn |
1.0±0.42 |
0.74 ±0.18 |
Table 1 Effect of cytokinins on shoot multiplication
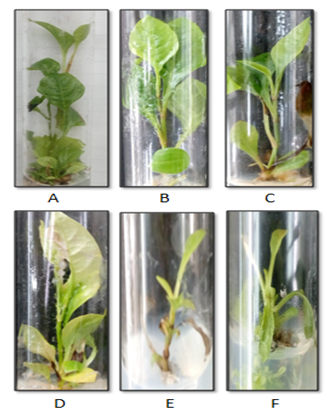
Figure 1 Effect of different cytokinin on shoot multiplication A) MS+1 mg/l BAP, B) MS+1.5mg/l BAP, C) MS+2mg/l BAP, D) MS+1 mg/l Kn, E) MS+1.5mg/l Kn, F) MS+2mg/l Kn.
Sahoo8 studied the role on BAP and Kinetin on shoot induction of Plumbago zeylanica, where BAP supplemented MS media produced more number of shoots that the kinetin supplemented MS media. Ceasar et al. 9 also reported similar kind of results in case of wild type Plumbago zeylanica plant. Gbadamosi & Egunyomi10 reported the highest shoot multiplication in MS medium containing NAA (0.01 - 0.05 mg/l) and BAP (2.0 - 4.5 mg/l). Dohare et al.11 reported highest shoot formation in the combination of 1mg/l BAP+ 1mg/l NAA and maximum length of shoot was recorded (5.38±0.99 cm).
Effect of different auxin concentration on callus induction
Induction of callus was observed an the cut end of leave explants after 2 weeks of incubation when modified MS (NaNO3 instead of NH4NO3) supplemented with 1.0 mg/l 2, 4-D and 1.0 mg/l NAA. Whereas media supplemented with the 1.0 mg/l 2, 4-D+ 0.5 mg/l BAP and 1.0 mg/l NAA+ 0.5 mg/l BAP did not show any callus response. An media supplemented with IBA showed root induction. Highest amount of callus induction was obtained in modified MS (NaNO3 instead of NH4NO3) supplemented with 1.0 mg/l 2, 4-D. Lubaina and Murugan (2012) reported that maximum callus proliferation when MS media supplemented with1 mg/l 2, 4-D along with 0.5 mg/l BA (Table 2), (Figure 2).
Media |
Callus Induction |
MS+1 mg/l 2, 4-D |
+++ |
MS+ 1 mg/l 2, 4-D + 0.5mg/l BAP |
- |
MS+ 1mg/l NAA |
++ |
MS+1mg/l NAA+ 0.5mg/l BAP |
- |
MS+1 mg/l IBA |
Root induction |
MS+1mg/l IBA+ 0.5mg/l BAP |
Root induction |
Table 2 Effect of auxins on callus induction
In this study, an attempt has been done to develop a method for the growth of Plumbago zeylanica by varying the cytokinin and auxin concentrations. Growth pattern was dependent on different growth hormones in both the shooting and rooting. 1.0mg/l BAP results in the highest shoot multiplication. MS media supplemented with 1.0mg/l 2, 4-D showed the faster callus induction rate. Mass propagation of this plant is feasible for field plantings to produce higher amount of roots which is the main source of plumbagin for the pharmaceutical industry. These in-vitro studies provide an efficient method for conservation and propagation this over exploited plant.
Our sincere thanks goes to Department of Biotechnology, Delhi Technological University for encouragement and providing necessary facilities during this investigation, Dr. Neelam Sharma, NBPGR, New Delhi for providing the plant materials.
The author declares no conflict of interest.

©2017 Roy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.