Advances in
eISSN: 2373-6402


Research Article Volume 7 Issue 4
1Department of Fisheries and Marine Bioscience Faculty of Biological Science and Technology Jessore, Bangladesh University of Science and Technology, Bangladesh
2Department of Aquaculture, Bangladesh Agricultural University, Bangladesh
3Department of Microbiology Jessore University of Science and Technology, Bangladesh
Correspondence: Abdulla-Al-Asif Department of Aquaculture Faculty of Fisheries, Bangladesh Agricultural University, Room No-137 Block- D Fazlul Haque Hall, Mymensingh, Bangladesh, Post code- 2202, Tel +8801716838294
Received: November 30, 2016 | Published: August 31, 2017
Citation: Rahman AMD, Rahman HMD, Yeasmin SM, et al. Identification of causative agent for fungal infection and effect of disinfectants on hatching and survival rate of Bata (Labeo. Bata) larvae. Adv Plants Agric Res. 2017;7(4):342-349. DOI: 10.15406/apar.2017.07.00264
Labeo. bata is one of the most important cultured fish. Intensive incubation leads to microbial overgrowth in L. bata eggs that hamper egg development hatchability and larval survivability. The aim of this study is to find out causes of mass mortality in L. bata eggs during peak breeding season from 10 March to May 2015 at Mafatema Fish Hatchery Jessore Bangladesh. Three concentrations of four chemical-formalin (10, 20, 30mg/L) malachite green (135mg/L) NaCl (123g/l) and methylene blue (135mg/L) treatment regimes and a control were compared for efficacy in treating L. bata eggs to prevent fungus and bacterial infection and improve hatch and survival rate of fry. Physicochemical and microbial characteristics of culture water were examined during the induced breeding of L. bata besides mycological examination of egg samples with trial of treatment of different types of disinfectant. The total bacterial count fluctuated between 3.6×108cfu/ml at initial time of incubation and 31.7×108cfu/ml after 3days of hatching. The infected fertilized egg by Saprolegnia sp. were appeared as tuft hairy like balls with a white cottony envelope that surround it which focally invaded the cytoplasm resulted in loss of the cytoplasm content and destructed envelops. Hatching rate (92.33±3.51%) of methylene blue at 1mg/L was significantly different with formalin at 10mg/L (78.0±5.29%) and control (72.33±5.51%) at.05 level of significance. Survival rate of malachite green at 5mg/L (87.33±6.51%) NaCl at 2g/L (91.00±3.00%) and methylene blue at 1mg/L (94.33±4.73%) had significant difference with control (71.00±8.89%) at.05 level of significance.
Keywords: causative agent, fungal infection, disinfectants, hatching rate, survival rate, labeo bata, larvae
In aquaculture venture and production the economic losses due to diseases are now important problems all over the world. It’s a major threat the sustainability of the aquaculture industry as a whole.1 Control of disease is too much difficult because of where fishes are farmed the whole production is depend on general environmental parameters. Among all cultured fish species L. bata is one of the most economic important fish species all over the world.2 Generally the fertilized eggs of carp are small spherical and demersal that hatch within 20±2 hrs at 28-30°C.3 In carp hatcheries mass mortality resulted from the microbial diseases.4 A physico-chemical condition in hatcheries as like as low dissolved oxygen low water temperature low circulated water high organic matter and high egg densities and were considered the main causes of fungal and bacterial attack of the egg.5,6 Some facultative bacterial strains may cause mass mortalities in eggs and sac larvae when present in adequate amounts.5 Mass mortalities of fish eggs are done by these bacterial strains which utilize oxygen and produce toxic metabolites.7 Some bacteria like Flavobacterium Sp. Pseudomonas Sp. Aeromonas Sp. and Vibrio sp. are easily to colonize and developed within hours after fertilization which mainly backed to water bacterial composition.8 Fungal infection is the most common scenario of financial loss in hatchery yield which mortality rate may reach to 80-100% in incubated eggs.9 Serious disease problems are happened due to aquatic fungi (Saprolegniales) in natural water supplies of fish hatcheries. Generally fungal diseases in farmed fishes are called saprolegniasis caused by fungal species in the genus Saprolegnia. Saprolegniasis is the one of the serious fungal infection in fish eggs.10 It causes major economic drawback in the fish farming industry infecting both fish and fish eggs.11,12 Generally Saprolegnia is inhibited naturally in all types of fresh water and it also found in dead fish eggs.12 From affected eggs the Saprolegnia can extent to live eggs via positive chemotaxi meaning that some chemical signal from the live eggs triggered the fungus to move towards them.13,14 Then primarily established the fungus makes further zoospores which affect more eggs. Therefore it is important continuously to eradicate dead eggs.15 Studies have shown that pathogenic for Saprolegnia species the thermal tolerance is similar to that of their host fish. In some other cases showed that render eggs in hatcheries susceptible to infection by the fungus. For example fungal spores are highly resistant to drying heat and disinfectants.6 So it is hard to exclude them from the intake water in fish-farms. Moreover poor water quality low dissolved oxygen rough handling high ammonia content water with minimum circulation stress decreasing temperatures and crowding factors are helped the fungus to survive and spread.6,13 Saprolegnia infections seem to come in turns different time. Often there seems to be no good reason for this. In the past this difficulties was resolved with the severely fruitful fungicide malachite green but in different case it has some negative impact.12 Eggs must be disinfected with suitable chemicals such as formalin malachite green methylene blue and salt can be used in fish hatchery eggs to defeat this economic loss. Formalin is a solution of 37-40% formaldehyde gas dissolved in water.16 Aqua culturists preferred to use therapeutic and prophylactic treatment for fungi external parasites protozoan and monogenic trematodes.17 Malachite green is widely disinfectant for fish eggs as a dip or flush. It has been used in controlling bacteria fungi protozoans and monogenetic trematodes on eggs fry and adult fish.18 Salt (sodium chloride) is harmless common substance which has antimicrobial characteristics.15 Control of fungus on fish and fish eggs malachite green is widely and effectively used but due to suspected teratogenicity that is potential carcinogenicity and mutagenic properties its use was utmost to the treatment of non-food fish that is egg or adult salmon held for spawning. The use of malachite green began in 1933 and it was one of the cornerstones used in treatment of fish against different range of parasites.19–22 It has been used widely by the aquaculture industry in Europe and all over the world for many years in the deficiency of an authorized veterinary medicinal alternative. It has some experimental proved effective in trial as a fungicide on farmed fish.15,19 In some cases malachite green works as a respiratory enzyme along with good disinfectants.23 Malachite green is compared as carcinogenic mutagenic (Committee on toxicity of chemicals in food consumer products and the environment 1999) and teratogenicity.9,12,22 Carcinogenic substances are agents capable of producing cancer. The present study is based on to identify the responsible causative agent for fungus infection and observe bacterial load in hatchery operation and short out the appropriate disinfectant that balanced with suitable hatching and larval survival rate.
The present research was performed during peak- breeding stage between 10 March to 15 May 2015 at Ma-fatema fish hatchery Chanchra Jessore and Central Laboratory and Laboratory of Fisheries and Marine Bioscience Jessore University of Science and Technology.
Sample collection
Aseptically sterilized glass container was used to collect the water samples (250 ml capacity). Sterilized screw cap tube was used to collected egg samples. Generally the samples were transferred to laboratory for examination within 2-3 hours of sample collection (Figure 1).
Bacteriological examination
Preparation of glass and plastic wares:At first Glass wares (petridishes test tubes L-sticks mortar conical flasks vials measuring cylinder etc.) were washed very nicely after that dry and sterilized at 170°C for 1 hour by a dry sterilizer. Time was maintained very carefully. The plastic materials were autoclaved at 121°C for 15 minutes.
Preparation of Physiological saline (PS):For laboratory examination an amount of 0.85g NaCl was weighed and kept in a measuring flask. It was then filled with distilled water to make the solution volume 100 ml. This was named as physiological saline (PS = 0.85% NaCl). Then the mixture was nicely mixed by vortex mixer. All the PS was autoclaved at 121°C for 15 min and kept at 4°C for future use.
Preparation of TSA plate:TSA media was used as a nutrient medium for bacteria culture. TSA medium was prepared by mixing at the rate of 40 g/l of distilled water in conical flask. Required amount of distilled water was measured in a cylinder. The mixture was heated on a hot plate for few minutes and then autoclave at 121°C. After autoclaving the whole prepared solution placed in clean chamber waited up to unheated to 60°C and then poured to sterile petridishes at an amount of 30 ml. After cooling and solidification all the TSA plates were turned upside down.
Bacterial isolation from water:A series of serial dilution of bacterial suspensions taken from the water samples was done up to 10-6 in sterile distilled water. A volume of 0.01 ml from suspension of 10-4 10-5 and 10-6 dilutions was placed in petridishes containing TSA. The outer cover of the petridishes was marked into three divisions corresponding to the designated serial dilution. Each petridishes was replicated three in number. The petridishes were kept incubated in an inverted position at 37°C for 24 hours (Figure 2). The optimum count of colonies from the designated division of each of the replicates was taken as mean ± standard deviation.
Mycological examination
The infected eggs were compressed with a drop of normal saline between two slide examined at low power magnification. Eggs with hyphae were taken for fungal isolation investigation according to Jafor and Saira (2013) in where generally using potato dextrose agar. Laminar flow air cabinet was used to isolate for avoid contamination. The agar plates were incubated at 25ºC temperature and fungal growth was observed after 3 days under microscope.
Effect of different disinfectants on the hatching rate of eggs and larval survivability
Definitive test with the fertilized eggs was carried out for the all breeding procedure in fishes. After fertilization 100 eggs were put in each of the three replicated thrice concentrations of formalin malachite green Sodium chloride and Methylene blue to determine the effective concentration that could reduce Saprolegnia sp. growth threshold concentration for sodium chloride formalin malachite green and methylene blue on the eggs as well as the hatching and survival rate of larvae. Three replication of control was also observed with the other treatments (Table 1 & Figure 3).
Name of chemical |
Concentration |
No. of Replication |
Formalin |
10mg/ L |
R1 |
R2 |
||
R3 |
||
20mg/L |
R1 |
|
R2 |
||
R3 |
||
30mg/L |
R1 |
|
R2 |
||
R3 |
||
Malachite green |
1mg/ L |
R1 |
R2 |
||
R3 |
||
3mg/L |
R1 |
|
R2 |
||
R3 |
||
5mg/L |
R1 |
|
R2 |
||
R3 |
||
Sodium chloride |
1mg/ L |
R1 |
R2 |
||
R3 |
||
2mg/L |
R1 |
|
R2 |
||
R3 |
||
3mg/L |
R1 |
|
R2 |
||
R3 |
||
Methylene blue |
1mg/ L |
R1 |
R2 |
||
R3 |
||
3mg/L |
R1 |
|
R2 |
||
R3 |
||
5mg/L |
R1 |
|
R2 |
||
R3 |
||
Control |
R1 |
|
R2 |
||
R3 |
Table 1 Treatment trials of fertilized eggs
Determination of hatching rate
Hatching of the eggs started after 20±2 hours of preliminary fertilization. The yolk sac absorption was observed by microscope that took place after 70±2 hours of hatching. When hatching of all eggs were completed the hatchlings were collected in a pot (dish) and counted by visual sight using magnifying glass and recorded. The hatching rate was determined by the following formula-
Estimation of larval survivability
The other context during the experiment was maintained in same condition. The total percentage of survival rate was determined by counting the total number of survived larvae after treatment. After accomplishing of the experiment at 5th day the number of total existed larvae in aquarium was counted individually for calculation of survival rate.
Data analysis
The results acquired from the experiment were performed to statistical analysis. Qualitative and quantitative analysis of data and facts were carried out. MS Excel and Graph Pad Prism 6 were used to store and analysis of all the data. MS Excel was also used for represent the tables and graphs achieved from different types of data. ANOVA test was done for the test of significance of hatching rate and survival rate of L. bata among different hatcheries and for the treatments data analysis we had used SPSS 16.0.
Bacteriological findings
Bacterial count fluctuated between 3.6×108 cfu/ml at initial time of incubation and 31.7×108 cfu/ml after 3 days of hatching. The total bacterial counts of water hatchery pool are shown in Table 2.
Name of Chemical |
Concentration |
Mean Initial Bacterial Load After 0 hrs |
Mean Bacterial Load After 96 hrs |
Formalin |
10mg/ L |
3.6×108 |
10.2×108 |
20mg/L |
7.2×108 |
||
30mg/L |
6.4×108 |
||
Malachite green |
1mg/ L |
11.2×108 |
|
3mg/L |
6.6×108 |
||
5mg/L |
5.9×108 |
||
NaCl |
1mg/ L |
6.4×108 |
|
2mg/L |
5.7×108 |
||
3mg/L |
4.9×108 |
||
Methylene blue |
1mg/ L |
9.2×108 |
|
3mg/L |
7.5×108 |
||
5mg/L |
6.0×108 |
||
Control |
31.7×108 |
Table 2 Counts of total viable bacteria in incubation bottle of L. bata
(Table 2). Counts of total viable bacteria in incubation bottle ofL. bata
Mycological findings
Tuft hairy like white cottony envelope was surrounded with the infected fertilized eggs in hatching containers. These eggs did not hatching and capitulated within 24-36 hrs. Microscopically the infected eggs showed highly branched hyphae with presence of zoosporangia. From these results the most correct classification of the fungus was Saprolegnia sp. (Figure 4).
Hatching rate observation
Hatching was begin after 20±2 hrs of fertilization. Fertilized eggs were treated with three concentrations of four disinfectants. In case of formalin malachite green NaCl and methylene blue treatment highest hatching rate was found 78.0±5.29% 83.67±3.51% 84.67±5.86% and 92.33±3.51% in concentration of 10mg/L 5mg/L 2g/L and 1mg/L respectively where hatching rate in control was found 72.33±5.51%. Hatching rate (92.33±3.51%) of methylene blue at 1mg/L was significantly different with formalin (78.0±5.29%) and control (72.33±5.51%) at.05 level of significance (Figures 5-9).
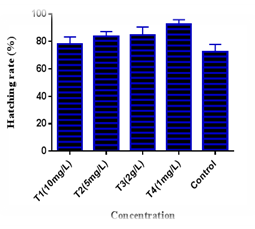
Figure 9 Effect of different disinfectant on the hatching rate of L. bata (T1-formalin, T2-malahite green, T3-salt and T4-methylene blue).
Observation of survival rate
After hatching survival rate was observed up to four days of fry. In case of formalin survival rate of fry was found 76.67±4.51% 63.00±9.64% and 46.00±6.24% at 10mg/L 20mg/L and 30mg/L respectively. Survival rate at 10mg/L is significantly different with 30mg/L treatment at.05 level of significance. In case of malachite green survival rate of fry was found 78.67±4.04% 84.00±7.21% and 87.33±6.51% at 1mg/L 3mg/L and 5mg/L respectively. No significant difference was found among three concentration of malachite green treatment. In case of NaCl survival rate of fry was found 79.67±6.02% 91.00±3.00% and 74.67±12.46% at 1g/L 2g/L and 3g/L respectively. Survival rate at 2g/L is significantly different with 3g/L treatment at.05 level of significance. In case of methylene blue survival rate of fry was found 94.33±4.73% 87.67±8.50% and 82.00±3.61% at 1mg/L 3mg/L and 5mg/L respectively. . No significant difference was found among three concentration of methylene blue treatment. Comparative survival rate of fry among formalin malachite green NaCl and methylene blue best concentration treatment with control was also observed. Survival rate of malachite green (87.33±6.51%) NaCl (91.00±3.00%) and methylene blue (94.33±4.73%) had significant difference with control (71.00±8.89%) (Figures 10-14).
In this present research the total amount of bacterial count oscillated between 3.6×108cfu/ml at initial time of incubation and 31.7×108cfu/ml after 3days of hatching. There was 8.8 times average escalated in bacterial population. It also found in the experiment that cfu/ml decrease with the increase of disinfectants concentration. Disinfectants reduce bacterial load through washing egg and other debris and treated water but due to continuous flowing water in incubation bottle bacterial load reduced as compare to control after four days but was not sufficient as bacterial load was 3.6×108in supply water.
Maximum bacterial load was found 4.2×1010cfu/ml after 24hrs of fertilization and the further load was 48×1010cfu/ml after 4days of incubation till hatching.24 For the common carp hatching procedure the bacterial load count was about 40.66x102 colonies ml-1 and after hatching rose to reach to 846.25×102 colonies ml-1. An another study found that the viable bacterial counts were 103/ml before hatching and rose to 106/ml after 2days of hatching in water pool of hatching unit of Gadusmorhua due to hatching egg debris and release of inorganic and organic substance.25 In another investigation found that the bacterial count was 102-105cfu/ml in rainbow trout rearing pond water.26 When the bacterial count more than 1800x102 colonies ml-1 embryos and hatchlings mass mortalities were recorded. The enormous growth of bacteria on eggs shell result in accumulation of lactic acid hypoxia and death of fish eggs.27,28 Beside excessive bacterial colonization may penetrate the egg shell or produce harmful toxic metabolites that could damage the chorion.8 In present research it has been confirmed by fungal isolation and microscopic studies where the infected L. bata eggs showed numerous fungal hyphae attached to the outer surface of the whole eggs besides pores in the inner envelope with focally invaded to the cytoplasm. This result is in agreement with the work of.29,30,31 Recorded that histopathological examination of infected eggs showed numerous fungal hyphae on the outer surface of the eggs and may penetrate the egg envelope and in some cases infected eggs showed germinated zoospores in the cytoplasm. This may return to the characterization of damaged dead egg or degradation of the egg envelope structural components by fungi released enzyme that facilitate hyphae penetrated across the egg envelope and accumulated in the cytoplasm.32,33
In this study fertilized egg were treated with three concentration of formalin malachite green NaCl and methylene blue. After four days treatment fungus infection was rarely found. Among three concentration of formalin treatment 10mg/L concentration was most effective and hatching rate and survival rate were found 78.0±5.29% and 76.67±4.51% respectively. 20mg/L and 30mg/L showed lower hatching rate and survival rate. Hatching rate and survival rate at 10mg/L (78.0±5.29% and 76.67±4.51%) was significantly higher than 30mg/L (40.3±6.11% and 46.0±6.24%) at.05 level of significance. Higher concentration of formalin treatment gives lower hatching and survival rate. Use of formalin in very small concentration effectively reduced fungal infection in the eggs and fry of Clarias gariepinus but it originated negatively on hatchability of the eggs and formalin is effective in treating saprolegnia.19,34–38 5mg/L concentration treatment of malachite green showed better result (hatching rate 83.67±3.51% and survival rate 87.33±6.51%) than other two lower concentration (1mg/L and 3mg/L) treatment. One hour treatment of malachite bath at the concentration of 4–5mg/l proved effective for the treatment of carp eggs infected with fungi Saprolegnia sp.36,39,40 Malachite green can prevent fungal infection of the eggs of Cyprinus carpioand tench which have been treated prophylactic ally.41
Widely the use of malachite green was restricted for the treatment of eggs of herbivorous fish and tench cause eggs of those fish species are very sensitive to malachite green along with bath treatment for salmonid fish eggs was described by Citek J& Willoughby LG39,42 Usually it was an international crime for its teratogenicity mutagenic and carcinogenic effect but still used in our country. NaCl at 2g/L concentration showed better Hatching rate (84.67±5.86%) followed by 1g/L (78.0±5.29%) then 3g/L (59.67±4.16%). 1g/L and 2g/L showed significantly higher hatching rate compared to 3g/L at.05 level of significance. 2g/L concentration salt treatment also showed higher survival rate (91.0±3.0%) as compared to 1g/L (79.67±6.0%) and 3g/L (64.67±12.66%). 2g/L showed measurably higher survival rate contrasted to 3g/L at.05 level of significance. This result is similar to;24 he found that sodium chloride at1.5 g/L for 60min. daily for 4days showed significantly higher hatching and survival rates.43 Different types of disinfectants are used to control fungal attack of eggs and to increase the survival rate of larvae. Among different disinfectants methylene blue is an important disinfectant. In the present study among three concentration of methylene blue treatment 1mg/L higher hatching rate (92.33±3.51%) and survival rate (94.33±4.73%) followed by 3mg/L (hatching rate 84.0±3.0% and survival rate 87.67±8.50%) then 5mg/L (hatching rate 76.67±4.16% and survival rate 82.0±3.61%). ANOVA test showed that there was significant differences with the percentage of hatching among treatments (P<0.05). Similarly some study were recorded that methylene blue solution highly increases the hatching rate of freshwater ornamental fish species.44 Usually the effect of methylene blue and sodium chloride on the bacterial load in the flush water with Nile tilapia (Oreochromis niloticus L.) fingerlings.45
In that study they found that the highest percentage survivability (99.6%) was found in 1mg/L of methylene blue treatment. Generally methylene blue is a redox dye type chemicals which are used to raises the oxygen consumption of cells. This means that the hydrogen to be oxidized is passed on to the oxygen. Each molecule of the dye is oxidized and minimize about 100 times per seconds. Thus while disinfection outcomes from this; methylene blue is also a magnificent chemical against met hemoglobin intoxication.46 There were significant differences in hatching and survival rate among chemicals and control. With the analysis of present study both methylene blue (1mg/L) and sodium chloride (2g/L) were most magnificent in reducing the bacterial load and counts as well as fungal infection along with in increasing hatching rate and survival rate of fry.
This study is focused on in case of disinfection treatment which is involved with the experiment of methylene blue at 1mg/L bath treatment every day for 4 days resulted significantly higher hatching rate (92.33±3.51%) and survival rate (94.33±4.73%). Hatching rate (92.33±3.51%) of methylene blue at 1mg/L was significantly different with formalin at 10mg/L (78.0±5.29%) and control (72.33±5.51%) at.05 level of significance. Survival rate of malachite green at 5mg/L (87.33±6.51%) NaCl at 2g/L (91.00±3.00%) and methylene blue at 1mg/L (94.33±4.73%) had significant difference with control (71.00±8.89%) at.05 level of significance.
None.
The author declares no conflict of interest.

©2017 Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.