Advances in
eISSN: 2373-6402


Research Article Volume 7 Issue 3
Department of Agronomy, Tamil Nadu Agricultural University, India
Correspondence: AICRP-WM, Department of Agronomy, Tamil Nadu Agricultural University, Coimbatore- 641003, Tamil Nadu, India
Received: October 31, 2016 | Published: June 19, 2017
Citation: Janaki P, Archana M, Priya SR, et al. Effect of herbicides on potato and their persistence in acid soil under semiarid tropical condition. Adv Plants Agric Res. 2017;7(3):272-277. DOI: 10.15406/apar.2017.07.00254
Field experiments were conducted to investigate the bioefficacy, phytotoxicity and field persistence of oxyfluorfen in/on potato and acid soil under Indian subtropical humid climatic condition. Both the herbicides gave good weed control at the recommended levels of application. Significant reduction in tuber yield was observed at higher rates of both the herbicides, though the phytotoxicity symptoms were not visible on potato crop. The dose of paraquat has significant influence on the population of bacteria and actinomycetes and fungal population was not affected by the dose. While the effect of paraquat on reducing the bacteria was significant upto 15 days, the fungi and actinomycetes population was revived on 15th day in all treatments. Oxyfluorfen and its doses have reduced the soil microbial community significantly on 7 and 15days after application. The residues of both the molecules were below the detection limit under the recommended rate of application. However at the higher rates of application, the oxyfluorfen residue was detected in soil and potato haulm and paraquat was above the detection limit only in soil at the time of harvest.
Keywords: oxyfluorfen, paraquat, potato, persistence, acid soil
Potato (Solanum tuberosum L.) is the world's leading vegetable crop and is grown in 79% of the world countries.1 It is an economically important staple food in both developed and developing countries because of its high yield potential and rich nutritive values. India is the second largest potato producing country in the world after China,2 with annual production of 37.3million tonnes from an area of 1.83million hectares. The problem of malnutrition and under nutrition can be largely solved if potato is accepted as a major food and not merely as a vegetable. It is a nutritious food containing practically all the essential dietary constituents. Carbohydrates are the major constituents of potato like in cereals and also contain proteins, essential nutrients and minerals like calcium, phosphorus and iron, and vitamins. Moreover, protein of potato is of high biological value.3 There is a great potential of exporting potatoes from India both for seed and table purposes to neighboring countries of South-East Asia and Middle East. Potatoes can even be exported to some of the European countries during March-May when fresh potatoes are not available in these countries. Hence good and best management techniques and methods are followed by the farmers to increase the production and productivity in India. Weeds, the undesirable plants compete with the early stage of potato establishment and reduce the tuber yield significantly. Though they are effectively managed either by cultural means, nowadays chemical method is highly preferred since it is quick, less laborious4 provides timely weed control and covers large area in a short time. Paraquat dichloride (1,1’-dimethyl-4,4’-bipyridylium dichloride) belongs to bipyridylium group is a fast-acting, non-selective contact herbicide absorbed by the foliage and destroys plant tissue by disrupting photosynthesis and rupturing cell membranes. It is highly immobile in soil and i resistant to microbial degradation under aerobic and anaerobic conditions.5 It does not undergo hydrolysis and photo degradation in aqueous solutions and the environmental dissipation occurs primarily by sorption to biological materials and soil clay particles and becomes environmentally inactive. Persistence of paraquat in soil environment is high with the field half- life of more than 1000days6,7 and can be degraded to non or less toxic compounds by the UV light, sunlight and soil microorganisms.8 However the strong adsorption to soil particles and organic matter limits its availability to plants and microorganisms with the soil Koc ranged from 8400 to 40,000,000.9 Increase in soil pH increases its sorption to soil particles and vice versa and the sorption is weaker in highly organic soils and remains active for long time and present up to 29days in soil with more than 98% organic matter.10 Since paraquat is not mobile in most soils,8 its bound residues persist for long time until transported in runoff along with sediments. Thus the risk of ground water contamination by paraquat is not high until directly applied to water bodies for aquatic weed control.11 Oxyfluorfen (2-Chloro-1-(3-ethoxy-4-nitrophenoxy)-4-(trifluoromethyl benzene) is the dominant substitution pattern second generation biphenyl ethers proton herbicide.12 introduced during 1987 and still research is continued on these groups. Oxyfluorfen is a contact herbicide used for the control of annual broadleaf and grassy weeds,13,14 as pre and post emergence and light is required.15 for its herbicidal activity in plants like paraquat. It degrades mainly by photolysis, showed moderate dissipation with the half life of 35days and its sorption is high in moist soils and also with high clay and organic matter content6 Since oxyfluorfen persist in soil and contaminate the aquatic surroundings through leaching and runoff,16 it is considered as highly persistent and toxic herbicide. Further the low water solubility (0.116mg/lit), vapor pressure (2x10-6mm Hg) and high soil organic carbon sorption coefficient (Koc) of 10,000ml g-1 for oxyfluorfen13 indicates its potential risk to the environment. Published studies reporting oxyfluorfen persistence in different soils showed a larger range of dissipation rates than the guideline studies. The field half life of oxyfluorfen applied to muck soils in Canada used for growing onions ranged from 30 to 103days. There are chances for bioaccumulation of residues if oxyfluorfen is used continuously and at higher doses.13,17 Recent decades, the paraquat has been used in potato as pre plant or early post emergence to control weeds and also as desiccant at the time of harvest of tubers. Similarly the oxyfluorfen has been used as pre emergence herbicide for weed control at potato field in India. Literature on paraquat and oxyfluorfen persistence in soil and crops are mostly confined to the field crops like rice, sunflower, fruits and plantations and very few have been reported in potato grown soils. Studying the interaction between the herbicides and soil micro-organisms is also of practical significance since the micro-organisms plays a unique role in removing contaminants and sustaining soil fertility. In view of the above facts, the present study was conducted to find out the phytotoxicity, persistence and residue of paraquat and oxyfluorfen in hilly acid soil under potato in tropical climate.
Field experiments
Field experiments concern to the oxyfluorfen and paraquat was conducted at the farm of Central Potato Research Station and farmer’s field at Kadanadu respectively in Ooty district, Tamil Nadu during autumn 2011-13. The experimental farms were located in Western hilly Zone of Tamil Nadu at 11°4’ N latitude, 76°6’ E longitude and an altitude of 2100m above MSL. Experiments were laid out in Randomized Block design and the treatments were replicated thrice. The size of each plot was 3m×2m. The potato variety Kufri jyothi was planted during October and harvested January. The test chemical (paraquat dichloride 24% SL and oxyfluorfen 23.5% EC) was applied at two different dosages (200 and 400g AI ha−1 for oxyfluorfen and 500 and 1000g AI ha−1 for paraquat) along with untreated control. While oxyfluorfen was applied as pre emergence, the paraquat was applied as early post emergence to control weeds in potato field using knapsack sprayer. Random sampling of soil from each treated and untreated control plots was done using a tube auger from 0-15cm depth at 6-7 places per plot. The soil samples were collected at the time of tuber harvest in all plots and processed as detailed by Janaki et al.13 The experimental fields soil is sandy clay loam in texture (Clay-23.5-25.2%, Silt-14.2-18.2% and sand (58.1-58.6%) and has Organic carbon 0.79-0.93%, pH 0.13-0.18, and EC 0.13-0.18dS m-1. Sampling of potato tubers and leaves was done at the time of harvest and stored under deep freezer at -15°C until proceeds to extraction and analysis. The normal weather conditions prevailed at the experimental locations was recorded daily during the cropping period. Maximum temperature in the cropping period ranged from 16.1°C to 21.9°C with a mean of 18.8oC. The minimum temperature ranged from 6.7°C to 12.1°C with a mean of 9.1°C. The relative humidity ranged from 67 to 97 per cent with a mean of 86 per cent in morning (07.22hrs) and 81 to 99 per cent with a mean of 92 per cent in the evening (14.22hrs).
Phytotoxicity rating and yield
Visual scoring for phytotoxic symptoms (crop discoloration, chlorosis, stunting, wilting, deformation and vein clearing) in potato was done on 3, 7, 15 and 30days after the application of herbicides to assess the injury to the crop using the scale from 1 to 10.18 At the time of harvest, potato tubers from each plot was weighed and expressed in t ha-1.
Microorganisms enumeration
A ten-fold serial dilution was made for each soil sample. Nutrient agar medium19 was used for the enumeration of total heterotrophic bacteria by the spread plate method in triplicate plates. Fungi population was estimated on Martin’s Rose Bengal agar medium20 and actinomycetes population was counted using Kuster’s agar21 medium. After the development of distinct colonies during incubations under suitable conditions, colonies were counted and the number of viable populations was expressed as colony forming units (CFU) per gram dry weight of soil.
Chemicals, reagents and soil
The certified reference standards of oxyfluorfen and paraquat and the test formulations namely oxyfluorfen 23.5% EC and paraquat 24% SL was supplied by Crystal Crop Protection Pvt. Ltd., (CCPPL), New Delhi, India. All the solvents used were of analytical grade and purchased from S.D. Fine Chemicals, Mumbai. For the preparation of stock solution of both the molecule and calibration standards and instrumental analysis, HPLC-grade solvents of E-Merck and 0.2µm filtered milli-Q (Millipore system, USA) water were used.
Instruments and Operating Conditions
The oxyflurofen residue was determined by the Thermo Gas chromatography (model GC8610) equipped with Electron Capture Detector (GC-ECD) as detailed by Janaki et al.,13 and the separation of compound was achieved using the mega pore capillary column. Under the given instrumental conditions, the oxyfluorfen was separated and detected at 4.92+0.2min. The paraquat was determined by the Double Beam UV-Visible Spectrophotometer of Varian (Model Cary 100) with 1cm Quartz glass cells and the intensity of blue colour was determined at the wavelength of 396nm.
Extraction and clean-up
Oxyfluorfen residue was extracted from soil and tubers by liquid-liquid partitioning with acetone13,14 and the filtrate was cleaned using anhydrous sodium sulfate and activated charcoal. The eluted organic layer was concentrated in rotary vacuum evaporator under reduced pressure and the sample was reconstituted in acetone for GC analysis. Paraquat dichloride in soil was extracted with H2SO4 under reflux and the filtered digest is percolated slowly through column of ambulate cation exchange resin. The sorbet paraquat dichloride is eluted using saturated ammonium chloride solution. Residue from potato haulm and tubers was extracted with the mixture of methanol and HCl22 by sonication and centrifugation. A portion of the eluted saturated ammonium chloride solution of soil extract and acidified methanol haulm and tuber extract was treated with alkaline sodium dithionite solution23 and the intensity of blue colour was measured differentially at 396nm using UV-Visible spectrophotometer.
Method validation and detection limits
Before proceeding to the persistence analysis of oxyfluorfen and paraquat in soil and tubers, the method was validated by fortifying control samples of potato tubers and soil with the known volume of different working standards of each herbicide separately. Method validation for oxyfluorfen was done as suggested by Janaki et al.13 and the quantification of residue was accomplished by comparing the peak response for samples with peak area of the standards. Paraquat dichloride extraction method was validated by spiking the blank matrices at different levels of 0.01, 0.05, 0.10, and 0.50μg mL-1 paraquat dichloride working standards. Spiked samples were processed to extraction, clean up and spectrophotometric determination as described for samples above. The concentration of paraquat dichloride in different matrix samples (mg kg-l) was calculated as suggested by Janaki and Chinnusamy.23
Recovery of oxyfluorfen and paraquat
Linearity of the herbicide molecules detection was assessed using the calibration curve of standard solutions. For both the molecules, the coefficient of determination was significant and is more than 0.99**. Accuracy of the methods adopted was evaluated in terms of recovery studies and the recovery ranged from 82.0 to 96.3 per cent for oxyfluorfen and 64.0 to 82.6 per cent for paraquat from different matrices (Table 1). Since the standard deviation between the replication data is less than 10 percent, the methods followed for extraction and detection of residues of both the molecules were found to be precise. Limit of Quantification was calculated by taking into account the herbicide concentration in control fortified samples, volume of extractions used, size of sample taken for analysis and the minimum amount of that can be determined without ambiguity. While the limit of quantification (LOQ) of oxyfluorfen was 0.006, 0.005 and 0.003μg/g respectively for field soil, potato tubers and plant tops, the LOQ of paraquat determination was 0.05μg/g for potato tubers and plant tops and 0.08μg/g for soil. The limit of detection (LOD) for oxyfluorfen was 0.001μg/g in all matrices and for paraquat, it ranged from 0.01 to 0.03μg/g of the across different matrices.
Matrix |
Concentrations Fortified (μg g-1) |
LOD |
LOQ |
||||
(μg g-1) |
(μg g-1) |
||||||
0.5 |
0.1 |
0.05 |
0.01 |
0.005 |
|||
Oxyfluorfen |
|||||||
Field soil |
94.8+1.26 |
87.0+2.30 |
89.2+3.01 |
86.5+2.14 |
79.4+4.61 |
0.001 |
0.006 |
Tubers |
89.4+2.01 |
83.0+1.85 |
89.8+4.15 |
88.2+3.50 |
83.6+3.12 |
0.001 |
0.005 |
Haulm |
93.4+1.92 |
82.0+2.91 |
90.5+3.12 |
91.1+2.91 |
84.2+5.24 |
0.001 |
0.003 |
Paraquat |
|||||||
Field soil |
77.8+5.42 |
79.0+3.12 |
84.0+5.21 |
BDL |
BDL |
0.03 |
0.08 |
Tubers |
82.6+4.61 |
76.0+4.07 |
74.2+4.35 |
BDL |
BDL |
0.01 |
0.05 |
Haulm |
83.0+3.15 |
79.0+6.13 |
76.0+3.46 |
BDL |
BDL |
0.01 |
0.05 |
Table 1 Average recoveries of oxyfluorfen and paraquat from fortified soil, potato tubers and plant tops BDL: Below Detectable Level
Phytotoxicity and bioefficacy of herbicides on potato yield and weed control efficiency
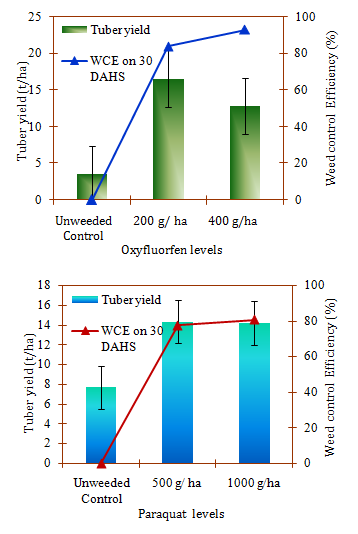
Phytotoxicity of the applied herbicides on the establishment of potato and growth parameters was studied on 7, 10, 15 and 30days after herbicide application. Application of oxyfluorfen or paraquat at different doses didn’t expressed visual toxicity symptoms on potato plant during entire period of crop growth. Paraquat being a contact herbicide kills or rigorously scorches only green foliage that comes in contact. The phytotoxicity symptoms have not been expressed by the plant, since paraquat was applied as early post emergence when potato seeds started germination.24 However, the tuber yield was significantly reduced by the herbicide and its dose (Figure 1). Significantly higher tuber yield of 14.33t ha-1 was obtained at the recommended paraquat dose of @ 500g/ha than at 1000g/ha though the weed control efficiency was high in this treatment. This could be ascribed to the toxicity of paraquat residue which was translocated to the tubers from soil through interfering in the source-sink translocation. Similar result of paraquat translocation to plants at minor amount has also been reported by JMPR24 especially under the conditions of low light intensity. Since, oxyfluorfen was applied as pre emergence; the chance for the toxicity expression on potato was not present. Similar to paraquat, significantly higher tuber yield was recorded at the recommended rate of 200g/ha than at 400g/ha. This showed that the higher rate of oxyfluorfen application have toxic effect on tuber growth and establishment though the visual symptoms were not shown by the plant. Detection of oxyfluorfen residue in soil at the time of harvest (Table 2) in the present study confirms the above results. The effect of oxyfluorfen and paraquat at different levels of application on potato tuber yield and weed control efficiency are presented in Figure 1. Significantly higher potato tuber yield at the recommended levels of both the herbicides could be ascribed to the higher weed control efficiency and lower toxicity of these herbicides residue on crop establishment and growth. Reduction in tuber yield at the higher levels of both oxyfluorfen (400g/ha) and paraquat (1000gha-1) was observed though they have given higher weed control efficiency. This confirms the toxicity of these herbicides residues to the potato crop at higher rates of application (Table 2). This could be attributed to the release of sorbet herbicide molecules from sorption sites at later period of crop growth in the present acid soils rich in organic carbon content Janaki et al.14 Decrease in tuber yield due to higher rate of oxyfluorfen was high when comparing the paraquat and substantiate that the toxicity of oxyfluorfen was more pronounced on potato yield than paraquat. This was established by the detection of its residues in potato haulm at the time of harvest (Table 2).
Matrix |
Oxyfluorfen Residue (μg kg−1) |
Paraquat residue (μg kg−1) |
||||
Control |
200 g ha-1 |
400 g ha-1 |
Control |
500 g ha-1 |
1000 g ha-1 |
|
Potato tuber |
BDL |
BDL |
BDL |
BDL |
BDL |
BDL |
Potato haulm |
BDL |
BDL |
3.46+0.75 |
BDL |
BDL |
BDL |
Soil |
BDL |
18.7+6.1 |
45.0+12.8 |
BDL |
BDL |
34.0+4.14 |
Table 2 Oxyfluorfen and paraquat residues in potato tuber, haulm and soil at harvest
BDL: Below Detectable Level
Effect of herbicides on microorganisms
Soil microorganisms are the biota that are first affected directly or indirectly by the toxic substances applied to any soil. They act as biomarkers to study the ecotoxicological influence of pesticides on soil system.25 In the present study, the bacterial population was significantly influenced by paraquat and oxyfluorfen doses during 7 days after herbicide spray and marked variation among the treatments was noticed up to 15days after application for paraquat only (Table 3), (Table 4). While the dose of paraquat has significant influence on the population of bacteria and actinomycetes, the fungal population was not affected significantly by the dose. The effect of paraquat on reducing the bacteria was significant upto 15 days, the fungi and actinomycetes population was revived and comparable among the treatments on 15th day. This showed that the paraquat and its dose of application have significant effect only on the bacterial population in the acid soil (pH below 5.6) while the fungi and actinomycetes population reached original population on 15thday. Tu et al.,26 also reported only the temporary suppression of nitrification organisms by paraquat and not the general microbial activities in high pH (>8.5) soils. Mewatankarn and Sivasithamparam27 reported that an increased fungal populations in soil by the paraquat application. The effects of oxyfluorfen on the soil microbial community showed that the population of bacteria, fungi and actinomycetes were significantly reduced by its doses on 7 and 15days after application. The low solubility of oxyfluorfen in water (0.116mg L-1 at 200C) might have retained it in soil surface13 and could have reduced the population of microorganisms. Bacteria, fungi and actinomycetes population was steady and stabilized on 30th days and the oxyfluorfen does not have any significant effect at later stages. Similar results were obtained by the Sireesha et al.28 due to the application of oxyfluorfen @ 0.15kg ha-1. The steady increase in microbial counts may be ascribed to their ability to temporarily mineralize and use herbicides as energy source. Sireesha et al.28 reported that after initial disturbance, there is generally a tendency to restore the original level quickly, as there is rarely a total exposure of soil micro organisms to biologically active concentration of herbicide. A change in species composition of soil microorganisms may occur after pesticide application but elimination of a single species is very unlikely.29
Dose (g/ha) |
Bacteria |
Fungi |
Actinomycetes |
||||||
(x 106 CFU g-1) |
(x 104 CFU g-1) |
(x 102 CFU g-1) |
|||||||
7th day |
15th day |
30th day |
7th day |
15th day |
30th day |
7th day |
15th day |
30th day |
|
200 |
28.02 |
29.28 |
29.23 |
19.26 |
20.48 |
29.52 |
9.63 |
12.46 |
15.33 |
400 |
17.35 |
20.28 |
27.33 |
14.26 |
15.48 |
26.85 |
6.63 |
8.13 |
14.5 |
Control |
32.35 |
33.28 |
30.56 |
25.59 |
27.48 |
27.19 |
16.63 |
16.48 |
17.36 |
SEd |
0.94 |
0.86 |
1.56 |
0.66 |
0.51 |
1.45 |
0.79 |
0.91 |
1.05 |
CD (5%) |
2.34 |
2.16 |
NS |
1.64 |
1.28 |
NS |
1.98 |
2.28 |
NS |
Table 3 Effect of oxyfluorfen on soil bacteria, fungi, actinomycetes (CFU g-1) in potato
Dose (g/ha) |
Bacteria |
Fungi |
Actinomycetes |
||||||
(x 106 CFU g-1) |
(x 104 CFU g-1) |
(x 102 CFU g-1) |
|||||||
7th day |
15th day |
30th day |
7th day |
15th day |
30th day |
7th day |
15th day |
30th day |
|
500 |
23.14 |
32.6 |
47.3 |
18.02 |
28.9 |
37.8 |
9.05 |
10.4 |
15.3 |
1000 |
18.02 |
33.9 |
48.1 |
13.41 |
29.6 |
38.5 |
7.61 |
10.8 |
15.9 |
Control |
31.15 |
48.5 |
48.5 |
23.52 |
30.3 |
39.5 |
11.21 |
9.4 |
15.3 |
SEd |
3.92 |
3.45 |
5.03 |
2.47 |
3.07 |
4.12 |
1.08 |
1.08 |
1.64 |
CD (5%) |
8.11 |
7.25 |
NS |
5.23 |
NS |
NS |
2.24 |
NS |
NS |
Table 4 Effect of paraquat on soil bacteria, fungi, actinomycetes (CFU G-1) in Potato
Persistence under field conditions
Application of each herbicide was done to the potato field at two rates viz., 200 and 400g AI ha−1 for oxyfluorfen and 500 and 1000g AI ha−1 for paraquat. The residue of both the molecules in the field soil, potato tubers and haulm were analyzed and the results are presented in Table 2. While the soil samples collected at post harvest from experimental fields contained 18.7 and 45.0µg kg-1 of oxyfluorfen residue across different doses, the residue of paraquat was detected only in higher dose (1000g AI ha−1) applied plot with the concentration of 34µg kg-1.This could be the results of stronger adsorption and deactivation of the paraquat residue by soil clay minerals and soil organic matter and its humic fractions.30 The detection of paraquat residue in soil at higher dose of 1000gha-1 might be due to the release of bound residues over a period of time. Further it could be attributed to the acidic condition of the soil as it is stable under acidic and neutral conditions.31 The presence of oxyfluorfen residues in soil under both the doses of application might be attributed to the weather conditions prevailed during the cropping period and its low solubility in water. While the low solubility retained it in soil surface, the low sunshine and temperature prevent the soil photolysis of oxyfluorfen and cleavage of the ether bridge which is the significant route of degradation.13 Besides, the high organic matter content might also retained it in soil for longer time as it have significant influence on the dissipation of oxyfluorfen from soil.14 Since oxyfluorfen is stable to hydrolysis at pH 4.0 to 9.032 and also not readily degraded by the microorganisms, it persist in soil until harvest of the crop. Though the residue is present in soil, the quantity detected is below 0.1mg kg-1 and could be the result of significant removal of oxyfluorfen from the field through runoff erosion because it is nearly insoluble in water, and therefore is unlikely to be mobile in most instances, unless the sorptive capacity of the soil is exceeded.6
Terminal residues of herbicides in potato tubers
At the time of harvest, potato tubers and leaves were collected from field and analyzed for the residues of paraquat and oxyfluorfen. It was found that the residue of both the herbicides was below detectable limit (0.001mg kg-1 for oxyflourfen and 0.01mg kg-1 for paraquat) in potato tubers; however the oxyfluorfen residue was detected in potato leaves and was found to 0.0035mg kg-1. Similar observations were recorded by Sondhia17 who reported 0.015 and 0.005µg g-1 residues of oxyfluorfen, respectively in green and mature onion samples collected on 60days of its application. It was reported that the oxyfluorfen have very little movement in treated plants and is not readily metabolized by the plants, however the residues in plants are generally low since it was not readily taken up by the roots.7 In the present study, though the oxyfluorfen resides are detected in potato leaves, the concentration is lower than the tolerance limit of 0.05mg/kg prescribed by the FSSAI33 for oxyflourfen in rice and groundnut, PMRA34 for dry onion bulbs and raspberries and USEPA35 for corms, taro and other vegetables. The residue of paraquat in potato tubers and leaves collected at the time of harvest were below the detection limit (0.01mg kg-1). Since it was applied as pre-emergence and might have well adsorbed by soil, its uptake by the plant is insignificant36 even at double the application rate of 1000g/ha. The concentration of paraquat in potato leaves was found to be below the tolerance limit of 0.2mg kg-1 potato prescribed by the FSSAI33 as well as 0.05mg kg-1 prescribed by the PMRA34 for the rooted vegetables.
Herbicides carry over effect on succeeding crop
Field bioassay method was used to conclude the bioavailable concentration of herbicides residue in the soil which can cause carry over injury to the succeeding crops and yield reduction. The carry over effect of oxyfluorfen residue from previous potato crop field on the germination of succeeding bio indicator species namely beans, its height and dry matter are presented in Table 5. It was found that the germination per cent, plant height, number of pods m-2 and yield of succeeding bean crop was not affected by the application of herbicides at different doses (200 and 400g ha-1). The herbicides applied at a higher dose did not leave any toxic residue in the field at the end of the cropping period. Similar to the oxyfluorfen, the germination of the succeeding crop of radish was not affected due to the application of paraquat dichloride. Different doses of paraquat also did not showed significant variation on the plant height and yield of radish (Table 5). Although residue of both the paraquat and oxyfluorfen was found within the safe limits prescribed by the FSSAI and PMRA, it is significant to observe that the residues of both herbicides were detected in reasonable concentration in soils after potato harvest. This would be results in immediate risks for the crops in succession viz., a possibility for the buildup of initial concentration higher above the tolerance threshold level and damage to the tolerant crop when the same chemical is continuously used for weed control.
Matrix |
Oxyfluorfen on Bean |
Paraquat on Radish |
||||
Control |
200 g ha-1 |
400 g ha-1 |
Control |
500 g ha-1 |
1000 g ha-1 |
|
Germination (%) |
95.0a |
95.67a |
91.7b |
92.1a |
95.3a |
89.6b |
Plant height (cm) on 30 DAS |
12.7a |
11.6b |
11.7b |
8.4a |
9.0a |
7.9a |
Yield (kg/ha) |
3761a |
3794a |
3795a |
6710a |
7560a |
5320b |
Table 5 Effect of oxyfluorfen and paraquat residues on growth parameters of succeeding crops
Values with same alphabet are comparable at 5% probability
In the present field study, both paraquat and oxyfluorfen gave good weed control at the recommended levels of application. Though the phytotoxicity symptoms were not visible on the potato crop, significant reduction in tuber yield was observed at higher rates of both the herbicides. Both the herbicides had significant effect on the microbial population when they were applied at higher rates. Residues of both the herbicides were within the safe limits prescribed by FSSAI and PMRA. Hence, these herbicides at recommended rate can be used for weed control safely in potato. However at higher rates of application, both oxyfluorfen and paraquat residues were detected in soil (above of 0.05µg g-1), the biomagnifications of residues of these herbicides in crop produce under continuous use should be investigated. Similarly, the continuous use of paraquat under long run in acid soil with high organic matter content may become a problem for groundwater quality which needs further investigation.
The authors are indebted to the Directorate of Weed Research, Jabalpur, India and Department of Agronomy, Tamil Nadu Agricultural University, India for providing necessary infrastructural facilities for the conduction of experiments.
The author declares no conflict of interest.

©2017 Janaki, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.