Advances in
eISSN: 2373-6402


Research Article Volume 6 Issue 4
1Horticulture Department, Al-Azhar University, Egypt
2Horticulture Research Institute, Agriculture Ministry ?Giza, Egypt
Correspondence: Helaly AA, Horticulture Department, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt
Received: December 27, 2016 | Published: March 2, 2017
Citation: Helaly AA, Goda Y, El-Rehim ASA, et al. Effect of irrigation with different levels of saline water type on husk tomato productivity. Adv Plants Agric Res. 2017;6(4):114-120. DOI: 10.15406/apar.2017.06.00223
This investigation was carried out in a private farm located at Shebeen El-Qanatir city, El-Qaliubiya governorate, Egypt during the two successive seasons (2011/2012 and 2012/2013 A.D.) to study the response of husk tomato plants (Physalispubescens L.) cv. (local variety) to different levels of saline water. To test the growth ability of salt tolerance with best fruit yield and their quality under saline condition. Plants was irrigated with salty water with concentration of 2000, 4000, 6000 and 8000ppm. The control pot was irrigated with tap water at the level of 260ppm. The results found that all water saline treatments significantly decreased the vegetative growth parameters & total chlorophyll content, NPK in husk tomato leaves, early and total yield. On the contrary, irrigation with saline water significantly increased sodium and proline contents in husk tomato leaves, fruit firmness, total soluble solids and total sugars as compared with the control. The fruit yield productivity was decreased, while the fruit quality was increased under saline irrigation.
Keywords: salinity, chlorophyll, yield, total sugars, NPK, Physalispubescens L
Husk tomato (Physalispubescens L.) is one of the most important vegetable crops in Egypt. The husk tomato belongs to the nightshade family (Solanaceae). The genus Physalis, established by Linnaeus in 1753, contains about 463 species but 100 species are well known and have more fanciful names such as husk tomato, golden berry, ground cherry, strawberry tomato, Cape gooseberry and pubescent ground cherry.1,2 Physalis has been known in Egypt since the sixteen centuries under the name of its varieties ‘Harankish’, ‘Halawyat’ and ‘El-Set El-Mestihya’. Because the fruit is covered in papery husk; giving it its name Husk tomato plants produce small orange fruits similar in size and shape to a cherry tomato. It is a hig.3 hly nutrition fruit; low in fat and contains no cholesterol or sodium. Husk tomato fruits provide an excellent source of the vitamin A and C, minerals (phosphorus and iron), protein, carotene, sugars and organic acids because of this they are a good choice for making health.2–4 Husk tomato (El-Set El-Mestihya, Harankish and Halawyat as Egyption people call) has been known in Egypt since long time ago by ancient of Egypt. Physalis is a very promising fruit. Recently, the economic importance of Physalis has risen, due to its high acceptance for local consumption, achieving great success in the African, Latin American and European markets.2,5,6 Many medicinal properties have been attributed to Physalis highly prized by Arab physicians as a medical plant for treating kidney diseases (as it purportedly disintegrated kidney stones) and urinary passage diseases. Recently, many studies have described the therapeutic applications and the pharmacological activity of the Physalis species as anti-parasitic, anti-viral, anti-neoplasic, antioxidant and anti-leukemic.5–7 Major problems still facing cultivation in new reclaimed lands, are salinity, drought and imbalanced nutrient elements.8 Salinity is one of the most important environmental constraints affecting more than 800 million hectares of arable land9–11 reported that the total salinity land is 953 ha which sharing 8% of the world area. The detrimental effects of high salinity on plants can be observed at the whole-plant level as the death of plants and/or decreases in productivity12 Salinity limits crops production, especially the sensitive ones.13 It affects morphological, physiological and biochemical processes, including seed germination plant growth and water and nutrient uptake.14 Percentage of dry weight, total soluble solids, and titratable acidity; content of reducing sugars, Cl, Na+, and various pericarp pigments; and electrical conductivity of the juice were higher in tomato fruits of saline-treated plants than they were in those of control plants, while the pH was lower.15 Salt stress affects some major processes such as germination, speed of germination, root/shoot dry weight and Na+/K+ ratio in root and shoot.16,17
Environmental stresses such as low temperature, salinity and drought limit crop productivity worldwide.18 Exposure to saline conditions is a detriment faced by many plants regardless of distance from large saltwater sources. According to the USDA nearly 30% of irrigated lands are of limited use because of salt intrusion, natural weathering or natural rainfall-based accumulation. Irrigation of plants or agricultural crops is the main cause of salt buildup in arid regions and areas where drainage in inadequate to remove excess salt.19,20 The saline area is three time larger than land used for agriculture. Total area under salinity is about 953million ha covering about 8% on the land surface. Excess salt in soil solution may adversely affect plant growth either through osmotic inhibition of water uptake by roots or through specific ion effect.21 The objectives of this study were to analyses the effect of saline water irrigation controlled on growth, yield and fruit quality of husk tomato plant.
The present study was carried out during the two successive seasons of 2011/2012 (first season) and 2012/2013 (second season) on husk tomato plant (PhysalispubescensL.) cv. local variety (Figure 1). Plants were grown in a private farm located at Shebeen El-Qanatir city, El-Qaliubiya governorate, Egypt. A pot experiment was conducted to investigate the effect of irrigation with various levels of saline water on husk tomato plants. The used water was brought from Karun Lake at El-Fayoum Governorate. The saline concentration of this water was about 30560 ppm salts which diluted with tap water to the required concentrations of 2000, 4000, 6000 and 8000 ppm. The control pot was irrigated with tap water at the level of 260 ppm. Chemical analysis of diluted drainage water is shown in Table 1 whereas physical and chemical properties of the soil which added to the pots are exhibited in Table 2. Pots were arranged in complete randomized design in three replicates. Each replicate consisted of seven pots. Seedlings were transplanted in pots as one transplant per pot (The pot contained wholes to drench the raised water, and its size was 50 cm in diameter and 80 cm in depth) filled with washed sand (30 kg dried sand /pot) and the experiment included 105 pots resulting from combination of 5 treatments within 3 replicate and every replicate consisted of 7 pots. Pots were irrigated with saline drainage water started after 10 days from transplanting date. Plants were irrigated with saline water twice per a week and each pot received 3 liters of water to maintain soil continuously moistened in the pot. EC ds/cm of the pots drained water was measured after every irrigation treatment, then the saline concentration of irrigation water was adjusted again compared with the main level of saline water before the next irrigation treatment. Each pot was fertilized with ammonium sulphate (10 g), super phosphate (8 g) and potassium sulphate (2.25 g). The amounts of fertilizers were divided into two equal parts; the first was employed after 4 weeks from transplanting date whereas the second one was added after 8 weeks later.
Salinity Levels |
PH |
EC ds/m |
Cations (meq/L) |
Anions (meq/L) |
||||||
Ca++ |
Mg++ |
Na+ |
K+ |
HCo3- |
HCO3- |
Cl- |
SO4- |
|||
Control at 260 ppm |
7.9 |
0.41 |
1.5 |
1 |
2 |
0.15 |
- |
3 |
1.5 |
0.15 |
NaCl at 2000 ppm |
8.88 |
3.13 |
3.25 |
6.75 |
25.3 |
0.4 |
- |
2.4 |
18.5 |
14.8 |
NaCl at 4000 ppm |
8.91 |
5 |
5 |
9 |
39.8 |
0.59 |
- |
3.6 |
32 |
18.79 |
NaCl at 6000 ppm |
8.98 |
7.5 |
6 |
17 |
57.6 |
0.97 |
- |
5.4 |
51.5 |
24.67 |
NaCl at 8000 ppm |
8.9 |
10 |
8.5 |
21 |
79.3 |
1.3 |
- |
7.5 |
74 |
24.6 |
Table 1 Chemical analysis of Karun Lake saline water after dilution with tap water
Seasons |
Physical Properties |
Chemical Properties |
||||||||||
Sand% |
Silt% |
Clay% |
Texture |
EC ds/m |
PH |
Soluble cation (meq/L) |
Soluble anion (meq/L) |
|||||
Na+ |
Ca++ |
Mg+ |
K+ |
Hco3- |
Cl- |
|||||||
First season |
97.8 |
1.3 |
0.9 |
Sand |
0.61 |
7.63 |
0.45 |
4.2 |
1.9 |
0.13 |
2.3 |
1 |
Second season |
97.2 |
1.5 |
1.3 |
Sand |
0.31 |
7.95 |
0.38 |
4 |
1.7 |
0.15 |
2.1 |
1 |
Table 2 Physical and chemical analysis of the pots soil before salinity experiments during2011/2012 and 2012/2013 seasons
Data were recorded from each plot as following
Vegetative characteristics :
Fruit characteristics
Statistical Analysis
All experiments were statistically analyzed in a complete randomized design with three replicates. Each replicate consisted of six plants. Obtained data were subjected to the analysis of variance procedure and means were compared by L.S.D. method at 5% level of significant according to.26
Vegetative characteristics
Physical parameters:Data about the physical parameters of vegetative characters are register in Figure 2. The results illustrated that increasing salinity levels from 2000 to 8000 ppm significantly decrease all physical parameters of husk tomato plants expressed as plant height, stem diameter, number of branches per plant and leaf area. The negative effect of salinity on plant growth and production is function of the relationship between dry mater production and water content (related to water uptake and transpiration) in plant tissue. The two components of this relationship, (dry mater and water), are independent and very much affected by conditions during growth such as (EC) in the root zone (or irrigation water). These explain the reduction in fresh and dry weight of fruit. As a general trend, irrigation with saline water affected negatively all plants growth parameters. Salinity is an environmental stress that limits growth and development in plants. Thus the irrigation with saline water at 2000, 4000, 6000 and 8000 ppm in this experiment induced changes in physical parameters of husk tomato plants. The irrigation with saline water from 2000 to 8000 ppm caused a significant reduction in plant physical parameters including plant height, stem diameter, number of branches per plant and leaf area. Salinity affects the crop during both the vegetative and the reproductive stages and therefore causes reduction in plant growth and development with low water potential in the root medium (osmotic effect), too high internal ion concentration (ion excess/toxicity) and nutritional imbalance by depression in uptake and/or shoot transport(ion deficiency)27 Osmotic effect resulting from salinity may cause disturbances in the water balance of the plant, including a reduction of turgor and an inhibition of growth, as well as stomata closure and reduction of photosynthesis 28 Also, these reduction effects of salt stress may be due to the effects of salts on the availability and uptake of water leading to decrease water content in the plant tissues which altered the metabolic processes inside the cells. Furthermore, increased salt content in the irrigation water may cause direct and indirect effects on leaf water relations and stomata closure which influence CO2 exchange and photosynthetic rate. Increased salt content in irrigation water may be directly toxic to plants, which intern, lowered carbohydrate accumulation in the plants29 Furthermore, negative effects of salinity have been attributed to disturbance in both protein assimilation30 mineral uptake and distribution activities ofgrowth hormones, enzymes activities and oxidative defense31 Also the reduction in previous parameters under the saline stress, may be caused by lower water uptake and reduced water transport to the leaves as reported by.32 Furthermore salinity induces osmotic and toxic effects leading physiological, morphological and biochemical modifications; it causes growth inhibition, lower photosynthesis and respiration, nutritional deficiencies and inhibition of protein synthesis33 These findings on the harmful effect of salinity on the whole growth performance of plants are similar to those reported by8 on tomato plant; 34 on Cape gooseberry (physalisperuviana)and35 on tomato plants.
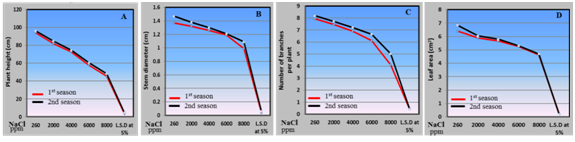
Figure 2 Effect of irrigation with saline water levels on plant height (a), stem diameter (b), number of branches per plant (c) and leaf area (d) of husk tomato plants during 2011/2012 and 2012/2013 seasons
Chemical parameters:The present study of chemical parameters of husk tomato plants, which included total chlorophyll content in leaves, macro and micro elements (N, P, K and Na) and proline under irrigation with saline water from 2000 to 8000 ppm, as shown in Figures 3 and 4 reflected two different trends. The first trend pointed to positive increase in sodium and proline percentage by increasing saline water levels from 2000 to 8000 ppm. Regarding the increase in Na percentage in husk tomato leaves by increasing saline water irrigation levels from 2000 to 8000 ppm may be attributed to the rise of pH level in the root zone resulted from salinity led to unavailability of potassium and calcium for the plant and also leads to accumulation of sodium inside the leaves 8,36 and.35 On the other side the present results about increment of proline percentage in husk tomato leaves due to the irrigation with saline water is in agreement with the results obtained with36 on tomato plants. However, many researchers demonstrated that plants accumulate proline in their leaves as a nontoxic and protective osmolyte under saline conditions.37 Proline accumulation under stress might occur due to an increase in pyrroline-5-carboxylate synthetase (P5CS), the rate-limiting enzyme in proline biosynthesis38 and a decrease of proline dehydrogenase (PDH) activity.39 The second trend showed a negative drop in total chlorophyll content, N, P and K in husk tomato leaves with every increase in saline levels. The negative effect of saline water irrigation on total chlorophyll content in husk tomato leaves in this study is attributed to salinity which indicates stress and damage to the photosynthetic apparatus. A decline in the level of photosynthetic pigments may be attributed to salinity-induced inhibition of chlorophyll biosynthesis.40 Salinity stress induced lower fresh weight and chlorophyll concentration of pumpkin genotypes. It has been reported that the typical symptom of salinity injury to the plant is the growth retardation due to the inhibition of cell elongation.41,42 Also, those harmful effects of salinity attributed to the inhibited effects on the activity of iron that reflect on reduction in rate of chloroplast structure and chlorophyll accumulation in tomato plant.43 From point of view salinity adversely affect the carbon fixation in photosynthesis, the lowest photosynthetic ability under salt stress conditions was due to stomata closure, inhibition of chlorophyll synthesis or due to decrease in the absorption of minerals needs for chlorophyll biosynthesis i.e. iron43 on tomato. Our results are in agreement with those obtained with8-12 on tomato.
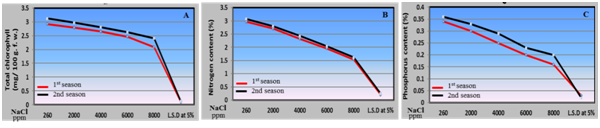
Figure 3 Effect of irrigation with saline water levels on total chlorophyll (a), nitrogen (b) and phosphorus (c) contents of husk tomato leaves during 2011/2012 and 2012/2013 seasons
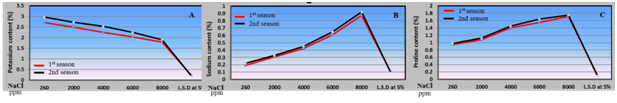
Figure 4 Effect of irrigation with saline water levels on potassium (a), sodium (b) and proline (c) contents of husk tomato leaves during 2011/2012 and 2012/2013 seasons.
The significant reduction in N, P and K percentage were noticed at the levels from 2000 to 8000 ppm, water salinity and this reduction increased gradually with increasing salinity. The least percentage of the previous elements in leaf tissues of husk tomato plants was obtained by using saline water at 8000 ppm. The reason of the reduction effect of saline water on nitrogen content in husk tomato leaves is due to the interaction effect between chlorine and nitrate. Chlorine accumulation decreased nitrate content in tomato and eggplant 44 Furthermore, the decreasein potassium content is due to an antagonistic effect between sodium and potassium.45
Moreover, the negative effect of water salinity on the percentage of N, P and K in husk tomato leaves may be due to the differences between osmotic pressure inside and outside the plants, i.e. around the root zoon and plant tissues35 on tomato. Our result is in agreement with those obtained with34 on Cape gooseberry.
Fruit characteristics
Physical parameters: Data presented in Figure 5 obvious that the irrigation with saline water levels induced change in the physical parameters of husk tomato fruits. Saline water irrigation levels from 2000 to 8000 ppm caused a reduction in some physical characteristics i.e. average fruit weight, size and fruit diameter, but it caused an increase in fruit firmness. The negative effect of saline water in average fruit weight, size and fruit diameter with very increase in saline water levels may be attributed to water uptake by husk tomato plants declines with the increase in salt concentration in irrigation water46 causing the decrease in fruit weight.47 Furthermore, reported that, tomato yield reduction was mostly associated with smaller fruit size. This was caused by a reduced enlargement rate during the exponential phase of fruit growth, which has been reported to be particularly sensitive to ionic and osmotic damages caused by ion accumulation in the plants throughout the growth season. Our results are in agreement with8 on tomato. In regarding to increase husk tomato fruit firmness in our investigation by saline water irrigation is due to increased salinity effect originating from reduced fruit water content due to adaptation of the plant to salinity. Same results were detected with8,48 on tomato.
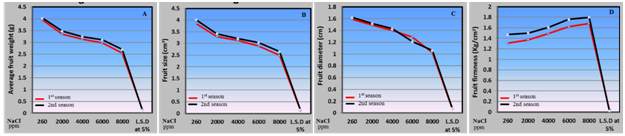
Figure 5 Effect of irrigation with saline water levels on average fruit weight (a), size (b), diameter (c) and firmness (d) of husk tomato fruits during 2011/2012 and 2012/2013 seasons.
Chemical parameters: Concerning the results of fruit Chemical characteristics which included total soluble solids (T.S.S.), total titratable acidity, vitamin C "ascorbic acid", total sugar, total carotenoids and dry matter under irrigation with saline water from 2000, 4000, 6000 and 8000 ppm reveal to irrigated with tap water as presented in Figure 6 & 7 reflected that a positive increase in all previous characters were obtained except total carotenoids.

Figure 6 Effect of irrigation with saline water levels on total soluble solid (T.S.S.) (a), total titratable acidity (b) and Vitamin C (ascorbic acid) (c) of husk tomato fruits during 2011/2012 and 2012/2013 seasons.
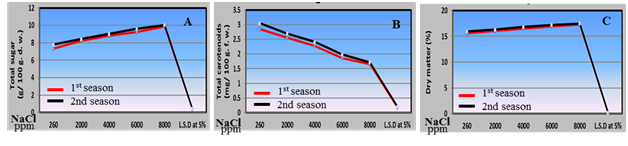
Figure 7 Effect of irrigation with saline water levels on total sugars (a), carotenoids (b) and dry matter (c) of husk tomato fruits during 2011/2012 and 2012/2013 seasons.
The enhancing contents of total soluble solids and ascorbic acid in husk tomato fruits by increasing saline water irrigation may be attributed to saline concentrations effect originating from reduced fruit water content due to adaptation of husk tomato plants to salinity.48 Also, in this study, husk tomato fruits grown under saline water irrigation show high titratable acidity, this may be attributed to the accumulation of organic acids in husk tomato fruits grown under salinity seems to counter balance the cation (K+ and Na+) excess respective to anions (Cl- and So4--) so maintaining fruits pH.49 On the other hand, the enhancing total sugar in husk tomato fruits by increasing salinity levels may be attributed to activity of sucrose synthesis enzymes intensified when plants cultivated under high salinity as reported by50 on tomato plants. In addition, increased total soluble solids, acidity and sugar content associated with saline irrigation may also be ascribed to concentration effects due to smaller fruit size as reported by.51 Our results are agreement with8 on tomato.
Yield parameters
Regarding the yield characteristics (number of fruits per plant, early and total yield per plot), obtained from husk tomato plants grown under various concentrations of saline water, results in Figure 8 indicated that saline water irrigation from 2000 to 8000 ppm caused a reduction in the previous characters.
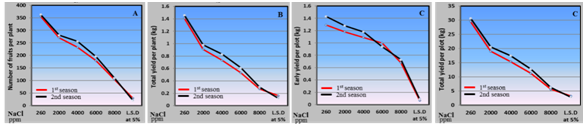
Figure 8 Effect of irrigation with saline water levels on number of fruits per plant (a), total yield per plant (b), early yield per plot (c) and total yield per plot (d) of husk tomato during 2011/2012 and 2012/2013 seasons.
The negative effect of the saline water attributed to the negative effect of saline water irrigation on leaf area, total chlorophyll content and NPK percentage in leaves which in turn built low yield of carbohydrate that consequently reduce the previous characters as mentioned by52,53 who reported that saline irrigation caused a reduced development of salinized plants which consequently manifested a reduced transpiration rate; salt-induced inhibition of the root pressure, which in turn would result in a reduced water movement into the xylem, contributing to lower water uptake by roots; and decreased soil permeability. Many researchers reported that, salinity is a major abiotic factor limiting plant growth and fruit yield .12 It induces osmotic and toxic effects leading physiological, morphological and biochemical modifications; it causes growth inhibition, crop yield reduction, lower photosynthesis and respiration, nutritional deficiencies and inhibition of protein synthesis.33 Our results are in agreements with and8,54 on tomato.
This experiment succeeded in providing evidence that husk tomato can grow well under 2000ppm of salinity with satisfying productivity and quality.55
Authors are grateful to Al-Azhar University, Faculty of Agriculture, Horticulture department, Government of Egypt for supporting this research project to carry out this work. The authors express their gratitude to, Prof. Dr. Abd El-Naem Said Abd El-Rehim and Prof. Dr. Mohamed Tarek Gaafar Al-Abd Professors of vegetable crops, Horticulture Department, Faculty of Agriculture, Al-Azhar Universityfor their Support, constructive suggestions, supervision, advice, sincere help and valuable guidance throughout the course of this study.56
The author declares no conflict of interest.

©2017 Helaly, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.