Advances in
eISSN: 2373-6402


Research Article Volume 6 Issue 3
1Institute of Environment and Agricultural Research, Research station of Farako, Burkina Faso
2Institute of Environment and Agricultural Researc, Environmental, Agricultural and Training Research Center of Kamboinse, Burkina Faso
3University Polytechnic Center of Dédougou, Burkina Faso
Correspondence: Wonni Issa, Institute of Environment and Agricultural Research, Research station of Farako, Burkina Faso, Tel +226-701-568-32
Received: January 28, 2017 | Published: February 6, 2017
Citation: Wonni I, Sereme D, Ouedraogo I, et al. Diseases of cashew nut plants (Anacardium Occidentale L.) in Burkina Faso. Adv Plants Agric Res. 2017;6(3):78-83. DOI: 10.15406/apar.2017.06.00216
Cashew’s (Anacardium occidentale L.) production in Burkina Faso suffers from several biotic constraints. The aim of this study was to inventory the diseases associated with the tree. Cashew orchards were prospected in the production belt of Burkina Faso including Hauts Bassins, Cascades, South-Western and, Center-Western regions. Four major diseases were observed including anthracnose (Colletotrichum gloeosporioides), Pestalotia leaf spot (Pestalotia heterocornis), bacterial leaf and nut spot (Xanthomonas citri pv. anacardii), and gummosis (Lasiodiplodia theobromae). The incidence of the diseases varied according to the locations; in addition, anthracnose was the most spread disease with high incidence. Gummosis was observed in all the locations and was the second threat to cashew production in South-West and West Center regions. The bacterial disease which affects all parts of the cashew tree was mainly observed in the Hauts Bassins region. No virus was detected during this survey. Altogether, the results of this study represent an important baseline data for the design and implementation of strategies for cashew protection in Burkina Faso.
Keywords: anacardium occidentale L, diseases, burkina faso
Cashew tree (Anacardium occidentale L.) is cultivated in more than 32 countries throughout the world and occupies the first place in regards to the production of nut.1 Africa ensures 40% of the total production out of raw nut whose 80% of this production are carried out in West Africa.2
Introduced into Burkina Faso from India in the early 1960s, cashew nut production generates incomes for more than 45.000 households with an annual production of nut over 35.000 tonnes/year.2 Nowadays, cashew is one of the most export-oriented horticulture crops in the country. Moreover, the number of processing units is increasing year by year throughout the country. In addition to its socio-economic importance, cashew tree plays an environmental part. It is used in afforestation schemes or as a fire protection barrier around forest demarcations.3 It also brings a protective vegetable cover while allowing intercalated food crops. The country counts about 45,000 growers among whom, 97% are located in Cascades, South-Western, Hauts-Bassins and, Center-Western regions, which have 17.500, 14.220, 10.000 and 2. 200 growers respectively.2 Cashew yield varies between 400 and 600 kg/ha.
Unfortunately, cashew is threatened by many biotic and abiotic constraints resulting in significant yield losses. Among biotic constraints, diseases and pests are the most damaging and compromise the cashew nut yield in terms of quality and quantity.4-15 Indeed, more than 12 diseases were reported to infect cashew tree worldwide. Anthracnose foliar blight, fruit rot, gummosis of twigs and trunk are often considered as the most relevant diseases causing severe damages across cashew producing countries.16 In Tanzania, four diseases including anthracnose, powdery mildew, leaf and nut black rust and bacterial leaf spot disease are responsible of extensive damage.17 According to Sijaona et al.,18 powdery mildew can cause yield losses ranging from 70 to 100%. In Brazil, anthracnose infection has drastically reduced cashew production by up to 40% in 2000.19 Study conducted by Afouda et al.20 highlighted four main diseases infecting cashew in Benin namely anthracnose, powdery mildew, red rust and pestalotia leaf spot.
Given this situation, the experience with diseases in other cashew producing countries (e.g., Mozambique and Côte d’Ivoire), demonstrate that an early action is of the utmost importance in controlling the disease and limiting production losses. Unfortunately, despite the socio-economic and environmental importance of this tree crop, data on cashew pathogens are scarce in Burkina Faso. Nonetheless, such country-specific data constitute, a prerequisite for the development of an efficient control strategy against the most economically important cashew diseases in the country. Since then, a broad approach that could accurately identify and analyse pathogens responsible for such diseases can contribute to design effective control measures.
The aim of the present study was to fill this gap by compiling an inventory of the diseases associated with different cropping stages of cashew in the main production areas in Burkina Faso.
Study areas
The inventory of cashew diseases was done over two years (from 2014 to 2016) in thirty orchards located in four major areas of cashew production in Burkina Faso. The orchards are sited in Hauts Bassins, Cascades, South-Western and Center-Western regions (Figure 1).
Sampling of infected organs
The sampling was performed in 2014 from july to september, in november in 2015 and mars in 2016 in orchards across the major production areas. Infected samples of twigs, leaves, flowers, and nuts were collected on ten trees chosen randomly following the diagonal of each orchard visited. Five to ten orchards were surveyed in each locality. For each sample, a number, the locality name, sampling date and geographical coordinates were noted. The samples were packaged in envelopes or plastic bags and transported in laboratory where they were stored at 4°C.
Diseases incidence was assessed on twenty trees distant to 10 m, selected randomly according to the diagonal of orchard during the sampling.
Incidence was estimated following the formula:
I(%)=Ai×100Ati
Where Ai is the number of trees infected by a disease and Ati, the total number of trees inspected.
Pathogens isolation and characterization
Isolation of fungi: The infected samples (branches, leaves) excepted for the seeds and flowers were cut into 3 mm pieces with sterile razor blade, surface-sterilized in 1% hypochlorite for 3mn, then placed between two glass slides on humidified blotter paper in Petri dishes. The samples were incubated at 28°C under alternating cycles of 12hours UV light and 12hours darkness for 7days. After incubation, fungi with different shape were observed under binocular microscope. Each fungi type was purified and transferred onto malt-agar medium and incubates for 5days. After incubation, the identification key of Mathur et al.,21 was used to identifying the different fungal species.
Pathogenicity test: young plants (6-10 leaves) of cashew were inoculated with fungi according to the method described by Touhami et al.22 The leaves, after disinfection with 70% alcohol, were spread with 120 ml inoculum concentrated at 105conidia/ml. Then plants were incubated under transparent plastic bag for 96h in order to maintain a high relative humidity favorable to infection. The re-isolation of fungi was performed from leaves after symptom apparition.
Isolation of bacteria: Bacterial isolations were performed from leaves as previously described by Wonni et al..23 Briefly, sections from the diseased leaf tissue were disinfected respectively in 75% alcohol, 1% hypochlorite and macerated in sterile distilled water. The macerate was diluted and streaked onto plates of LPGA (Yeast extract, Peptone, Glucose, Agar) medium supplemented with cephalexin at 40mg liter-1, kasugamycin at 20mg liter-1 and propiconazol at 50mg liter-1. Plates were incubated for 3 to 7days at 30°C. A single suspect bacterial colony was purified from each tissue sample, then incubated for 48h. After incubated, the purified strains were characterized by biochemical tests in comparison with Xanthomonas citri pv. citri reference strain isolated by Zombré et al.24
Biochemical tests: The purified isolates were identified by conventional bacteriological methods, including Gram test, Kovac’s oxidase test and oxidation-fermentation (OF), hydrolysis of starch and gelatin tests. The biochemical traits obtained were compared with those reference strain of Xanthomonas citri pv. anacardii.
Pathogenicity test: The bacterial strains having 100% biochemical traits identical to reference strain of Xca were tested. The young leaves of the same vegetative growth from 9months old plants, after disinfection, were infiltrated with bacterial suspension concentrated at 108CFU/ml using a needleless syringe. Plants were incubated for 3days under transparent plastic bag and watered regularly for symptom expression. Re-isolations were done from leaves infected in accordance with the Koch postulate.
Virus diagnosis: Data on virus infecting Anacardiaceae family such as mango and cashew are still poorly documented. However, during our prospection, leaves samples were collected according to the presence of symptom (yellowing, mottle, mosaic, and necrosis) on cashew leaves. Asymptomatic leaves were sampled and use as control. Leaves sampled were placed in plastic bags to avoid all contact between leaves and the hand. Each sample was numbered and placed in cooler with pre-chilled ice packs unless it is being transported to the laboratory and stored at -20°C. Based on bibliographic research and symptoms observed on cashew leaves, Plum pox virus (PPV) was suspected to be the causal pathogen. Indeed, PPV belonging to the Potyvirus genus within the family Potyvirida, induces similar symptoms on plum tree causing important damages. So, a pair of Potyvirus universal primers, NIb2F and NIb3R, previously designed25 were synthetized and used to amplify the genome after RNA extraction.
Total RNA extraction: Total RNA was extracted from leaves using trizol according to the manufacturer’s recommendations (Invitrogen). RNA of Yam mosaic virus (Potyvirus genus) was also extracted from infected leaves and used as control. RNA extracts were then eluted in a final volume of 30μl using RNAse free water and stored at 4°C.
RT-PCR: cDNA were synthetized using the "SuperscriptII RNase H-Reverse Transcriptase" (Gibco BRL, Life Technologies) kit according to the manufacturer’s recommendations with 1µl of total RNA extracted and the reverse primer (0,5µM) NIb3R (5’-TCIACIACIGTIGAIGGYTGNCC-3’). PCR was performed with cDNA using the pair of primers Nib2F (5’-GTITGYGTIGAYGAYTTYAAYAA-3’) and Nib3R; and the GoTaq DNA Polymerase (Promega) in 50µl of the master mix as described by Zheng et al.26 DNA purity and concentration were estimated by electrophoresis on 1% agarose gel containing ethidium bromide.
Data analysis: Microsoft Excel software was used to enter data. ANOVA and Duncan’s multiple comparison tests were performed with SPSS 20 software. QGIS version 2.12 software was used to build the map from the GPS coordinates.
Diseases inventoried
Several symptoms and physiological disorders were recorded in the surveyed orchards. These symptoms include leaf and fruit spots, rust, mosaic and/or chlorosis. These infections had variable incidence depending on the localities. However, anthracnose was the most spread disease with a high incidence in all production areas (Figure 1).
Anthracnose symptoms were observed on the leaves (Figure 2), inflorescences and nut (figure 3]. Symptoms were characterized on young leaves of cashew seedlings or adult plants by water-soaked spots initially and become orange-brown to light-reddish with age and sporulation of the fungus. In severe cases leaves and fruitlets become totally blighted and drop. Pestalotia symptoms on leaves were characterized by the transparent and discolored aureoles in the beginning, then, becoming reddish brown, rounded and necrotic (Figure 4A).
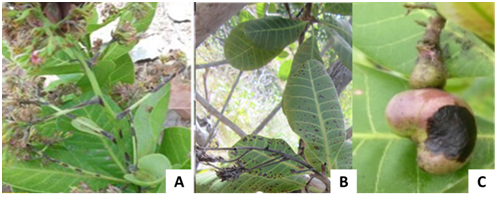
Figure 3 Symptoms of anthracnose
Symptoms of red rust resulted in one or more red, circular powdery spots on the upper side of the leaves (Figure 4C). Powdery mildew was observed with very low incidence in surveyed orchards (Figure 4B). It is characterized by whitish or grayish-whitish mildew colonies observed on the upper surface of leaves.
The symptoms of bacterial leaf spot were characterized by angular necrosis spots located near leaf veins (Figure 5A), canker on twigs (Figure 5B) and oily stains becoming necrotic on the nut (Figure 5C).
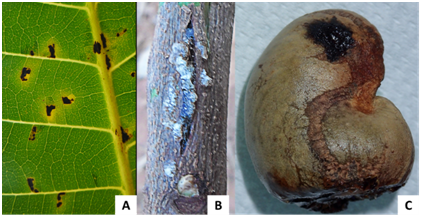
Figure 5 Bacterial disease symptoms.
(A) Black spot on leaf,
(B) Stem canker,
(C) Black spot on nut.
The gummosis was characterized by gum exudation on the trunk and branches (Figure 6A and B) which is the most visible symptom after yellowing and leaf drop. It was observed in almost all surveyed orchards and affected both young and old trees.
Algae attacks on the trunk and branches (Figure 6C) were observed in different orchards with very low incidences. We observed also yellowing, necrosis and mosaic on leaves similar to symptoms caused by viruses.
Pathogens associated with symptoms
About fifteen fungal and bacterial isolates were identified based on morphological, biochemical and biological characteristics. The table 1 reports the list of the fungi associated with different symptoms observed.
Fungi identified: Ten fungal species were identified in all the surveyed sites. There are: Colletotrichum gloeosporioides, Pestalotia heterocornis, Cladosporium sp, Aspergillus niger, Botrytis cinerea, Fusarium sp., Curvularia sp., Penicillium sp., Rhusopus sp. and Cephaleuros virescens. However, C. gloeosporioides (Figure 8), Pestalotia heterocornis (Figure 9) and Cephaleuros virescence had the high frequencies (Data not showed). Pathogenicity tests of C. gloeosporioides and P. heterocornis showed similar symptoms to those sampled following inoculation.

Figure 7 Physiological disorders or virus symptoms (A, B et C) in comparison with Plum Pox Virus leaf symptoms (D)
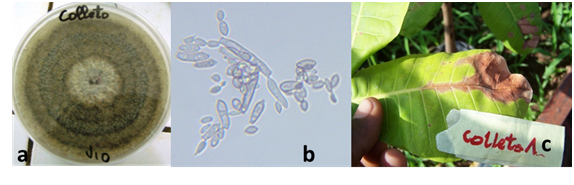
Figure 8 Characteristics of C. gloeosporioïdesisolates
(a) Mycelium morphological aspect and (b) conidia
(c) Symptoms induced after inoculation
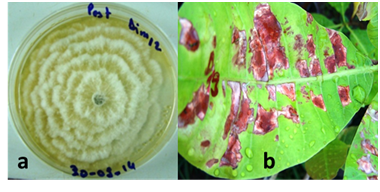
Figure 9 Characteristics of Pestalotia heterocornis isolates:
(a) Mycelium morphological aspect,
(b) Symptoms induced after inoculation.
Leaves |
Twigs and branches |
Flowers |
Nut and apple |
|
Symptoms |
Necrosis, drying, red powdery spots, burning others spots (black,pale green to yellow), angular spots |
Necrosis, drying, black spots |
Rut, necrosis, black spots, exudates, cankers |
|
Pathogens |
Colletotrichum gloeosporioides, Pestalotia heterocornis, Cladosporium sp, Curvularia sp, Fusarium sp, Aspergillus niger, Botrytis cinera, Cephaleuros sp., |
Colletotrichum gloeosporioides, Fusarium sp, Aspergillus niger, |
Colletotrichum gloeosporioides, Pestalotia heterocornis, Botrytis cinera, Penicillium sp, Fusarium sp, Aspergillus niger, |
Colletotrichum gloeosporioides, Penicillium sp., |
Alternaria sp., Aspergillus flavus, Xanthomonas citri pv. anacadii, Erwinia sp |
Fusarium solani, |
Aspergillus niger, Aspergillus flavus, Curvularia trifolii, |
||
Alternaria brassicae |
Alternaria sp., Phoma sp., Rhusopus sp., Botrytis cineréa |
Table 1 Pathogens associated with infected samples of cashew
Bacteria: Two genus of bacteria, namely Xanthomonas and Erwinia were identified base on biochemical and pathogenicity tests. In comparison with reference strain of Xca, all the isolated strains induced typical symptoms of bacterial leaf spot. No symptom was observed after the infiltration of leaves with Erwinia sp. strains.
Virus: About twenty samples with yellowing, necrosis, mottle and mosaic symptoms (Figure 7) were collected across surveyed cashew orchards. However, the Potyvirus universal primers used in this study did not allowed to detect virus.
Cashew pathogens diagnosis highlighted the prevalence of diseases such as red rust (Cephaleuros virescens), anthracnose (Colletotrichum gloeosporioides), pestalotia leaf spot (Pestalotia heterocornis) and gummosis of twigs and trunk [Lasiodiplodia theobromae (Pat.) Griffon & Maubl.] in the different sites spread throughout the main growing regions of the cashew in Burkina Faso. However, infection severity was variable. This result underlines the major fungi which can threaten the productivity of cashew if control measures are not undertaken to limit their spreading inside the country. Similar results were found by different authors in Benin and Tanzania.1-18 Anthracnose is by far the most important disease in the field, leading to significant yield losses. Our results are consistent with those of Freire et al. 10 and Araújo.4
Some studies revealed that fungi are responsible of gummosis.6 According to this author, up to now, despite the important damage induced by gummosis disease on cashew, no efficient measure is available to control it. Many authors reported an increase in gummosis severity in all north-eastern producing states in Brazil.6,7-17 Thus, it appears necessary to consider this threat very seriously in cashew orchards in Burkina Faso.
Other foliar infections, namely pestalotia leaf spot (Pestalotia heterocornis), red rust (Cephaleuros virescens) and powdery mildew (Oidium anacardii F. Noack), also occur but with almost negligible consequences in cashew orchards. Our results are in close conformity with those reported by Ghini et al.12 but in contrast with previous reports1 wherein disease incidence ranging from 73 to 90.0% was recorded for red rust in Benin. However, it is important to mention that precipitation, humidity and temperature are key factors for the fungus dispersal and infection.
Penicillium sp., Aspergillus Niger, Botrytis cinerea, Curvularia sp and Fusarium sp. isolated from cashew foliar organs were recorded on flowers and leaves in Nigeria Otuonye.19 These fungi would be associated with cashew tree as saprophytic parasites but could appear pathogenic under favourable environmental conditions. Thus, in the context of climate change, it is necessary to take preventative action in order to prevent or lessen these serious threats to cashew production.
Xanthomonas citri pv. anacardii previously characterized by Zombré et al.,25 was identified in the Hauts-Bassins region in Burkina Faso. This causal agent of bacterial leaf and fruit spot has already been described by many authors in Tanzania, Brazil, and Benin.18-3,11-1
Mango tree is a host of X. citri pv. anacardii, pathogen responsible of bacterial leaf and fruit spot (Zombré et al.25 In addition, X. citri pv. mangiferaeindica, the major threat to mangoes production which can caused up to 100 % yield losses25 was reported to infect cashew.24 In Burkina Faso, cashew is often growing in association with mango tree. Altogether, this means that X. citri pv. anacardii and X. citri pv. mangiferaeindica could be a serious constraint to cashew production in Burkina in the future.
The prevalence of these pathogens on cashew orchards in Burkina Faso would partially justify the decrease of cashew yield these last years. Considering the potential which represents the production of cashew nut and the possible development of the culture in the country, more investigation on bioaggressors infecting this crop tree is needed. Such a study will have the merit to inventory the nematodes infecting cashew which was not take into account in the present study but whose presence and its incidence were announced in Nigeria.2
Most plants infected with a virus can’t be cured. So, once a plant becomes infected, it is usually remains infected throughout its life. In this scenario, even no virus was detected in the present study using Potyvirus specific primers, it proves to be necessary to conduct more exhaustive investigation, focusing on the use of metagenomics approaches as novel tool of detection such as recommended by several authors.16,14,20 That is also true for the identification and characterisation of the others bioaggressors as well as nematodes, bacteria, and fungi. Interestingly, today, it is clear that Next-Generation Sequencing (NGS) as an emergent tool, presents an enormous potential to better characterize diseases by providing extensive knowledge on plant pathogens at a large scale.
In addition, the present study does not investigate on the real impact of each diseases on the cashew nut yield. Consequently, further investigations are needed to clarify this aspect in cashew orchards in Burkina Faso and in West Africa in general.
Finally, the diversity of pathogens recorded on cashew in Burkina Faso is noteworthy. This may offer a potential for the development of sustainable control strategies against the economically important cashew diseases.
To our knowledge, this is the first study compiling an inventory of cashew diseases and highlighting their importance in Burkina Faso. The study thus, draws an attention to cashew growers in the world of the emerging threat to cashew crop. Right now, it is necessary to take care of the rigorous application of the regulation relating to plant material exchanges in order to avoid the introduction and/or the dissemination of cashew diseases.
This article is the result of the combined efforts of a multidisciplinary team of phytopathologists (IW, DS, IO, AIK, ID, LO) and entomologist (SN) who contribute to elaborate the protocol, fungi identification (IO, AIK, IS), bacteria characterization (IW, LO) and virus diagnosis (DS). IW drafted the manuscript which has been reviewed by all co-authors.
This work was supported by the GIZ/Matching Fund and CORAF through the cashew project. We thank sincerely Dr Vianney TARPAGA for for facilitating the funding for sampling and pathogen isolation.
Authors have declared that no competing interests exist.

©2017 Wonni, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.