Advances in
eISSN: 2377-4290


Research Article Volume 8 Issue 3
1Manchester Royal Eye Hospital, UK
2Manchester Vision Regeneration (MVR) Lab, NIHR/ Wellcome Trust Manchester CRF, UK
3Manchester Academic Health Science Centre and Centre for Ophthalmology and Vision Research, University of Manchester, UK
Correspondence: Paulo E Stanga, Consultant Ophthalmologist & Vitreoretinal Surgeon, Professor of Ophthalmology & Retinal Regeneration, Manchester Royal Eye Hospital, Oxford Road, Manchester, M13 9WL, UK, Tel +441612765580
Received: November 20, 2016 | Published: May 1, 2018
Citation: Gil-Martinez M, Pastor-Idoate S, Yau K, et al. Wide-field fundus autofluorescence and swept-source-optical coherence tomography imaging after successful retinal detachment surgery. Adv Ophthalmol Vis Syst. 2018;8(3):139?143. DOI: 10.15406/aovs.2018.08.00288
Purpose: To increase our understanding of cellular and structural changes in the retinal pigment epithelium (RPE) and the neuroretina (NR) after successful rhegmatogenous retinal detachment (RD) surgery using Wide-Field (WF) Fundus Autofluorescence (WF-FAF) and Swept-Source Optical Coherence Tomography (SS-OCT).
Methods: Retrospective, non-interventional study. Twenty-three (23) patients (24 eyes) who underwent successful RD surgery were imaged using WF colour and FAF and 1,050nm SS-OCT and the images analysed. Pre and post-operative Best Corrected Visual Acuity (BCVA) and post-operative visual symptoms were recorded.
Results: The mean age of patients was 57.2±13.8 years old. The macula was preoperatively OFF in 13 eyes and ON in 11 eyes. Eleven eyes (45.8%) underwent pars plana vitrectomy (PPV), endolaser and intraocular injection of gas. Eight eyes (33.3%) underwent PPV with cryotherapy and 5 eyes (20.8%) underwent cryobuckle (CB) surgery. Post-operative visual acuity (post-VA) improved in 100% of patients. Patterns of abnormal autofluorescence were present in 91.7% of the macula OFF RD patients and in 37.5% of the macula ON RD. SS-OCT macular abnormalities were present in 84.6% of eyes with Macula OFF RD and in 27.3% of eyes with macula ON RD. Patients were asked for post-operative symptoms and these were recorded.
Conclusion: Post-operative WF-FAF and SS-OCT imaging allows the non-invasive assessment of anatomical changes in the RPE and the neuroretina at both cellular and tissue level, as well as the functional status of the RPE, thus explaining visual outcome and residual post-operative symptoms. Further studies are required to investigate pre-operative biomarkers of post-operative improvement in vision and residual visual symptoms.
Keywords: rhegmatogenous retinal detachment, retinal surgery, anatomical results, post-operative visual outcome, imaging, biomarkers, wide-field fundus autofluorescence, swept-source-optical coherence tomography imaging, oct, retinal pigment epithelium, neuroretina
Rhegmatogenous retinal detachment (RRD) is a common ocular disease with an incidence that ranges between 6.3 and 17.9 /100,000 people/year. Certain groups are reported to have higher prevalence, such as men, highly myopic eyes (>6 diopters), aphakia, pseudophakia, and adults between the ages of 60-70 years.1,2 The pathogenesis of RRD is a complex process resulting from inherited and/or age-related changes in the structure of the vitreous body and the vitreoretinal interface, which can predispose to the formation of retinal tears with the subsequent passage of vitreous fluid into the subretinal space.3 RRD surgery is the most commonly performed vitreoretinal surgery, with primary success rates of repair range between 64 to 91%.2 However, in spite of the currently achieved high anatomical success rate, a variable percentage of patients can experience incomplete visual recovery. It is traditionally believed that preoperative visual acuity (pre-VA) is the strongest pragmatic indicator of postoperative visual acuity or outcome (post-VA).4 Post-VA may be also related to the type and extent of the RRD, macular involvement and duration of the RRD.5,6
WF-FAF is a non-invasive test that provides funduscopic images based on stimulated emission of light from lipofuscin (LF). This resulting autofluorescence is dependent on the renewal of photoreceptor outer segments and is potentially affected by the balance between accumulation and clearance. Increased phagocytosis of photoreceptor outer segments gives rise to hyperautofluorescence.7 Some authors have used FAF to assess the unintentional displacement or “slippage” of the retina after standard PPV. Postoperative hyperfluorescent lines parallel to the retinal vessels seem to highlight the RPE that was preoperatively under the vessels, thus suggesting retinal displacement and a possible effect on visual outcome following successful RRD surgery.8 SS-OCT utilises longer wave-length and faster scanning speeds than FD-OCT and is therefore less subject to intraocular light scatter by the vitreous and the RPE, amongst others, as well as registration errors due to eye movement, allowing for higher resolution images.9,10 New technological advances in retinal imaging such as WF-FAF and SS-OCT may increase our understanding of the relationship between anatomical, ultrastructural and functional changes at the level of the RPE and the neuroretina and post-VA. The preoperative detection of biomarkers of visual outcome in these patients could prove advantageous to patients and hence our interest in first looking at post-operative markers. The purpose of the present study is to try to correlate the postoperative anatomical, at both cellular and tissue level, and functional status of the neuroretina and the RPE on WF-FAF and SS-OCT with post-operative visual outcome.
An observational retrospective study was performed between September 2013 and January 2014 at the Manchester Royal Eye Hospital and the Manchester Vision Regeneration Lab at the Wellcome Trust Manchester Clinical Research Facility. Included patients were non-consecutive. However, we have only included in this study consecutive patients that underwent all of the described imaging studies. All necessary institutional approvals and patient consent were obtained. All included patients were 18 years of age or older and had undergone successful surgical repair of an acute and uncomplicated RRD. Patients with proliferative vitreoretinopathy, silicone oil tamponade, lens opacity or without follow-up were excluded. Pre and postoperative Best Corrected Visual Acuity (BCVA), pre and post-operative visual symptoms, time between onset of symptoms, pre-operative extension of the RRD in number of quadrants affected, type of surgical procedure, macular status on slit lamp biomicroscopy were recorded and analysed.
WF-FAF was performed using the Optomap P200Tx imaging system (Optos PLC, Dunfermline, Scotland) during the routine postoperative follow-up examinations (15 days to 60 months). All images were captured using a 532nm wavelength light for excitation (green range) and a 570nm to 780nm light (yellow-orange-red-range) for detection and analysed using the proprietary software Optos V2 Vantage. SS-OCT (DRI 1-Atlantis, Topcon Corp, Japan) was performed in all the patients during the same visit as the WF-FAF assessment. The 1,050 nm wavelength DRI-OCT was set to a single line scan, with a resolution of 1024 and a length of 12mm. The longitudinal single line scan was centred on the fovea and travelled across the optic nerve. All the images were analysed by 3 independent observers.
Twenty-four (24) eyes of 23 non-consecutive patients affected by RRD were studied. Table 1 summarizes the data and variables analysed in this study. Of these twenty-four (24) eyes, 54.16% (n=13) presented a macula OFF RD at the time of the surgery and 45.83% (n=11) presented a macula ON RD. Out of the 24 eyes; 79.2% (n=19) underwent PPV with gas tamponade and 20.8% (n=5) underwent CB procedure. In the group of eyes treated with PPV; endolaser was used in 57% of eyes (n=11) and cryotherapy in 43% (n=8). C3F8 intraocular gas tamponade was used in 7 eyes and SF6 intraocular gas tamponade was used in 12 eyes.
Case |
Sex |
Age |
preop VA |
Postop VA |
Macula status |
Type of surgery |
OCT |
WF-FAF |
Symptoms |
Follow-up |
1 |
M |
82 |
1 |
0.3 |
Off |
23G PPV +Endolaser+C3F8 |
ERM |
Macular changes |
Blurred vision |
36 M |
2 |
M |
45 |
2.7 |
0.6 |
Off |
23G PPV + Endolaser+SF6 |
EZ |
Macular changes |
Blurred vision |
1 M |
3 |
M |
56 |
0.7 |
0.3 |
Off |
23G PPV +Cryotherapy+SF6 |
EZ |
Displacement |
Blurred vision |
1 M |
4 |
M |
70 |
2.7 |
0.1 |
Off |
23G PPV +Cryotherapy+C3F8 |
EZ |
Displacement |
Blurred vision |
3 M |
5 |
M |
60 |
1 |
0.4 |
Off |
23G PPV +Cryotherapy+SF6 |
ORF |
Uninterpretable |
Blurred vision |
15 D |
6 |
F |
60 |
1 |
0.6 |
Off |
23G PPV +Cryotherapy+SF6 |
CSMO |
Demarcation area |
Blurred vision |
21 M |
7 |
F |
82 |
0.8 |
0.5 |
Off |
Cryobuckle |
ORF |
Demarcation area |
Blurred vision |
60 M |
8 |
F |
35 |
1 |
0 |
Off |
Cryobuckle |
EZ |
Demarcation area |
Blurred vision |
26 M |
9 |
F |
46 |
2.7 |
0.6 |
Off |
Cryobuckle |
EZ |
Demarcation area |
Metamorpsia |
14 M |
10 |
M |
62 |
0.2 |
0.1 |
Off |
23G PPV +Cryotherapy+C3F8 |
EZ |
Displacement |
Metamorpsia |
1 M |
11 |
M |
43 |
2.7 |
1.5 |
Off |
23G PPV + Endolaser+C2F6 |
ORF |
Demarcation area |
None |
1 M |
12 |
M |
64 |
0.7 |
0.1 |
Off |
23G PPV +Endolaser+C3F8 |
Normal |
Normal |
None |
5 M |
13 |
F |
42 |
0.7 |
0.6 |
Off |
23G PPV +Cryotherapy+SF6 |
Normal |
Displacement |
None |
15 D |
14 |
F |
63 |
0.1 |
0 |
On |
23G PPV +Cryotherapy+C3F8 |
Normal |
Macular changes |
Better vision |
1 M |
15 |
M |
26 |
1 |
0 |
On |
Cryobuckle |
Normal |
Displacement |
Diplopia |
1 M |
16 |
F |
51 |
0.2 |
0.9 |
On |
23G PPV +Cryotherapy+ |
IRF |
Normal |
Blurred vision |
12M |
Endolaser+SF6 |
||||||||||
17 |
F |
61 |
0.3 |
1.3 |
On |
23G PPV +Endolaser+C3F8 |
EZ |
Uninterpretable |
Blurred vision |
15 D |
18 |
M |
52 |
0.9 |
0.1 |
On |
Cryobuckle |
Normal |
Normal |
None |
15 M |
19 |
M |
68 |
0.1 |
0 |
On |
23G PPV +Endolaser+SF6 |
IRF |
Normal |
None |
25 M |
20 |
F |
56 |
0.1 |
0 |
On |
23G PPV + Endolaser+SF6 |
Normal |
Normal |
None |
16 M |
21 |
F |
56 |
0.2 |
0 |
On |
23G PPV +Criotheratpy+SF6 |
Normal |
Normal |
None |
15 D |
22 |
M |
67 |
0.1 |
0 |
On |
23G PPV +Endolaser+SF6 |
Normal |
Uninterpretable |
None |
15 D |
23 |
F |
54 |
0.1 |
0 |
On |
23G PPV +Endolaer+SF6 |
Normal |
Uninterpretable |
None |
15 D |
24 |
M |
71 |
0.7 |
0.3 |
On |
23G PPV + Endolaser+SF6 |
Normal |
Displacement |
None |
15 D |
Table 1 Characteristics of the Sample
BCVA and symptoms
For statistical analysis, BCVA was converted to logMar values. BCVA improved in the majority of patients (91,6%, n=22) and the difference was statistically significant (p=0.029 t-Student paired 2 tails). Two (2) patients went on to develop a post-operative worsening of BVCA because of the development of macular epiretinal membrane (ERM) (n=1) and macular oedema (MO)(n=1). We found that 39.1% (n=9) of patients were asymptomatic, 4.3% (n=1) showed improved vision, 43.5% (n=10) reported monocular blurred vision, 8.7% (n=2) experienced monocular metamorphopsia and 4.3% (n=1) complained of binocular diplopia.
WF-FAF changes
WF-FAF was performed on 91,7% (11/1) eyes with a macular OFF RRD and on 37.5 % (3/8) of eyes with a macula ON RRD. A normal WF-FAF pattern was observed in 62.5% (5/8) of eyes macula ON RRD and in 8.3% (1/12) in the macula OFF RRD. Out of 70% (14/20) of eyes that showed an abnormal WF-FAF pattern: 30% (6/20) of eyes showed retinal displacement observed as hyperfluorescent lines parallel to the retinal vessels (Figure 1A), 15% (3/20) showed macular changes (Figure 1B) and 25% (5/20) showed a well-defined hypo (long term) or hyper-fluorescence (early follow –up) delimiting the area that pre-operatively corresponded to the RRD (Figure1C). Normal autofluorescence (Figure 1D) was observed in 30% (6/20) of the eyes and corresponded mainly with asymptomatic patients (30%, n=6). Twenty percent (20%) (4/20) of the images could not be interpreted because of the presence of gas obscuring the area of the previous RRD at the time of imaging.
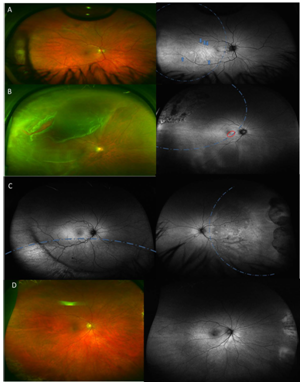
Figure 1 A WF-Colour image, Optomap P200Tx imaging system (Optos PLC, Dunfermline, Scotland) of a symptomatic patient without any obvious changes on biomicroscopy. A-II. WF-FAF image of the same patient showing retinal displacement (blue arrows). B WF- Colour image of a patient with RRD. B-II WF-FAF of the same patient showing a pattern of linear hyperfluorescence in the macular area (red circle) associated with blurred vision. C Area showing hyperfluorescent demarcation line corresponding with SRF in a patient treated with cryobuckle surgery. C-II Posterior pole area of hypoautofluorescence in a patient experiencing metamorphopsia after RRD. D Normal looking WF-Colour image. D-II WF-FAF in an asymptomatic patient after RRD surgery. (Blue dotted line indicates the area of detachment was present in each figure).
SS-OCT findings
Macula OFF RRD was associated with macular abnormalities on SS-OCT in 84.6% (11/13) of eyes and Macula ON RRD in 27.3% (3/11). Perhaps as expected, a normal SS-OCT appearance was highly associated with eyes with macula ON RRD 72.7% (8/11) compared with macula OFF RRD 15.4% (2/13). SS-OCT showed changes in the neuroretina in 58.3% (14/24) of all eyes. We classified these changes into: 1. Changes in ellipsoid zone (EZ) (Figure 2A) (50% of eyes (7/14)), 2. Inner Retinal Folds (IRF) (Figure 2B) (14.3% of eyes (2/14)), 3. Outer Retinal Folds (ORF) (Figure 2C) (21.4% of eyes (3/14)), 4. Macular Oedema (7.1% of eyes (1/14)) (Figure 2D)) and 5. ERM (7.1% of eyes (1/14)). Nine eyes (37.5%) did not present any alteration on SS-OCT and these were coincident with asymptomatic patients (Figure 2E). The presence of changes in ellipsoid zone, Inner or Outer Retinal Folds on SS-OCT scans was associated to the suffering of post-operative visual symptoms such as metamorphopsia or blurred vision see Table 1.
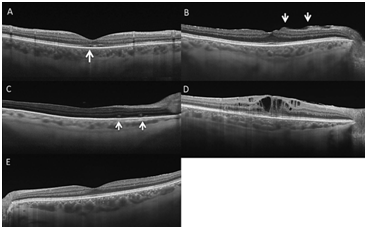
Figure 2 Swept Source Optical Coherence Tomography (DRI 1-Atlantis, Topcon Corp, Japan) showing the different patterns that we have identified. A Disruption of ellipsoid zone (white arrow showing disruption of IS-OS in subfoveal area). B Inner retinal folds arrowed. C Outer Retinal folds arrowed. D Macular oedema. E Normal OCT.
Correlation between SS-OCT and WF-FAF
We have observed that 50% of the total number of eyes (n=10) presented changes on both WA-FAF and OCT. We observed that WF-FAF changes affected mainly the mid and peripheral RPE (50%, n=10), while the SS-OCT changes were macular. We found a positive correlation of presence/absence on both SS-OCT and WA-FAF in 70% of eyes (n=14). However, 20% of eyes did not show abnormal patterns neither on WF-FAF nor SS-OCT.
Although, RRD surgery has significantly improved the rate of anatomical success over the past years, a successful anatomic result does not always correlate of post-VA improvement or absence of persistent visual symptoms. This occurrences has been attributed to failure of retinal receptor regeneration or their post-operative misalignment.11 The development of new imaging techniques has improved our knowledge of different retinal pathologies, allowing us in some cases, to evidence ultrastructural retinal changes and to correlate them with symptoms. WA-FAF is a new imaging technique which allows a claimed 200° view of the RPE autofluorescence pattern; this imaging technology being perhaps best suited for imaging fundus changes in RRD. WF-FAF imaging evaluates the functional status of the RPE by mapping the spatial distribution of RPE lipofuscin. This imaging technique has been successfully used to post-operatively assess changes in the treated areas following SB surgery.12 However, although the main source of FAF is the LF from the RPE, recent studies have reported that there might be other sources of hyperfluorescence after RRD such as subretinal deposits and macrophages which have phagocytosed the photoreceptor outer segments.7
Machemer demonstrated, in experimental models, how the RPE undergoes morphological changes within hours after the development of a RRD and how this event is followed by the degeneration of the outer and inner segments of the photoreceptors.13 Recent studies have confirmed that the separation of the neurosensory retina and the RPE can lead to photoreceptor cell death secondary to the loss of metabolic interaction with the underlying RPE and choroid.14 In our study, we showed the presence of a hyper-fluorescence demarcation line in early post-operative follow-up that could progress to hypo-fluorescence. This hyper-fluorescence could be secondary to the persistence of SRF that can lead to the consequent atrophy of the RPE, and which would then be visualised as hypo-autofluorescence in later follow up visits. We observed demarcation lines associated to macula OFF RD in eyes that underwent delayed surgery. The demarcation line could be explained by the presence of SRF for a prolonged time and the subsequent hypo-autofluorescence secondary to RPE/Photoreceptor cell death or loss of their interaction. Witmer et al.,15 suggest that WA-FAF could be useful for monitoring the reabsorption of SRF after scleral buckling procedures, as a hyper-fluorescent area of SRF that reduces with time after successful treatment can be noted. Eighty percent (80% (n=4)) of patients who underwent SB with cryotherapy presented changes on WA-FAF. This could be perhaps secondary to the persistence of SRF for a longer time compared to PPV with subsequent RPE changes and loss of photoreceptors.
A possible association between hyper-autofluorescence and persistence of SRF has been suggested by Spaide et al.16 These authors reported that a delay in the reabsorption of SRF was found to be associated with the accumulation of cream-coloured aggregates on the posterior surface of the retina. Robertson et al.,17 analysed SRF and reported the presence of macrophages containing complex melanosomes, lipid particles and membranous cytoplasmic bodies, theorizing that these were derived from photoreceptors.18 These cellular by-products are suggested to be hyper-autofluorescent on FAF.16 Some authors suggest that rods recover better than cones following successful retinal reattachment surgery.19 WF-FAF is necessary to image changes in the pattern of mid and peripheral RPE autofluoresence due to the prevailing presence of rods in these areas.
Other authors have described unintentional retinal displacement as a morphologic phenomenon that may occur after PPV for RRD. Retinal displacement has been associated with the pre-operative extension of the RRD and with the use of gas tamponade. It has also been described as more frequent in patients with macula OFF RD. The occurrence of displacement has been reported to vary between 52.5% and 62.8%, depending on the series.8,17 However, in our study, only 25% of the patients showed retinal displacement. This is a significantly lower percentage compared with previous reports and could be secondary to the surgeon’s personal surgical techniques. Pandya et al.,20 suggested that displacement could contribute to poor visual prognosis. However, we did not observe this correlation in our series.
Our study did show a correlation between presence of symptoms and changes on WF-FAF as 92.85% of the symptomatic patients demonstrated an abnormal pattern on WA-FFA and 70% of the asymptomatic group showed a normal pattern. Furthermore, we observed a good correlation between patients with macula OFF RRD and changes on WA-FAF, as 85.7% of them showed changes compared with only 37.5% of patients with macula ON RRD. See Table 2 of the images evaluated, only 16.6% (n=4) could not be interpreted. This could have been due to the time when we obtained these images; 15 days post-operatively. At this point in time during the follow-up, some patients still presented intraocular gas that obscured part of the fundus and this did not allow for the interpretation of the images, especially of the superior retina and RPE. However, other studies suggested that maybe it should be necessary to wait until all the intraocular gas has reabsorbed to successfully perform the WA-FAF.21
Macula status |
WF-FAF |
SS-OCT |
||
Off |
Normal |
8.30% |
Normal |
15.4 |
Abnormal |
91.70% |
Abnormal |
84.6 |
|
On |
Normal |
62.50% |
Normal |
72.7 |
|
Abnormal |
37.50% |
Abnormal |
27.3 |
Table 2 Correlation between changes on WF-FAF and SS-OCT in eyes with macula ON and OFF RD
Disruptions of the ellipsoid zone/external limiting membrane have been previously described using FD-OCT technology and associated with poor visual prognosis.5 Though the presence of changes in ellipsoid zone, Inner or Outer Retinal Folds on SS-OCT scans in our study was associated with the suffering of post-operative visual symptoms such as metamorphopsia or blurred vision See Table 1, it is difficult to assess in this pilot and retrospective study if there is a specific pattern that can be associated to a specific post-operative symptom. There is no published report that identifies the superiority of either posterior pole FAF or FD-OCT as a means of identifying markers of successful visual outcome after successful RRD surgery. Previous authors reported that FAF and FD-OCT abnormalities did not anatomically correlate in the majority of the cases. These authors also suggested that FD-OCT can demonstrate acute changes in the neuroretina, while posterior pole FAF indicate the presence of ultrastructural changes in the RPE.22 However, there is no previous study correlating WF-FAF and SS-OCT.
We did find a correlation between abnormalities in WA-FAF and SS-OCT in 70% of patients (n=14) and no correlation in 30% (n=6) of them. Our study is the first to look at the combined use of WF-FAF and SS-OCT for the assessment of the anatomical and functional status on the macular neuroretina and the macular, mid and peripheral RPE and to show the superiority of the combined use of WF-FAF and SS-OCT over the combined use of posterior pole FAF and FD-OCT. In conclusion, our study shows that WA-FAF and/or SS-OCT could be useful tools to explain some of the functional changes and patient symptoms following successful RRD treatment by showing abnormal patterns that may not be clinically visible. This is a Pilot Retrospective Study with several limitations such as a small sample size and short follow up. However, we believe this study has proved the need for a larger and prospective study designed to demonstrate differences between groups and to look at pre and post-operative imaging biomarkers of prognosis for visual outcome. Its potential findings could allow for a better understanding of the effects of RRD on visual function and the development of treatment strategies to improve post-operative visual functional results.
This research was facilitated by the Manchester Vision Regeneration (MVR) Lab at NIHR/Wellcome Trust Manchester CRF by providing the imaging equipment and clinical facilities and the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network by providing clerical assistance.
The author declares that there is no conflict of interest

©2018 Gil-Martinez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.