Advances in
eISSN: 2377-4290


Research Article Volume 4 Issue 4
1Department of neurosugery and ophthalmology, Ivano-Frankivsk National Medical University, Ukraine
2Department of Human Anatomy, Ivano-Frankivsk National Medical University, Ukraine
3Department of ophthalmology, Rogatyn central district hospital, Ukraine
Correspondence: Moyseyenko Nataliya M, Department of neurosugery and ophthalmology, Ivano-Frankivsk National Medical University, Ukraine, Tel +380677566341
Received: April 28, 2016 | Published: June 28, 2016
Citation: Nataliya MM, Oksana ZY, Halyna LM. Ultrastructure features of damage to the cranial part of the optic nerve at traumatic short-time compression of its orbital unit in the experiment.
The role of compression factors on the formation of secondary degenerative factors and anti- and retrograde parts of the optic nerve in traumatic injuries has not been fully studied.
Purpose: Was to clarify ultrastructure features of the optic nerve cranial part damage at traumatic short-time compression of its orbital part in the experiment.
Methods: The simulation of traumatic injury of the optic nerve was performed on 11 rabbits weighing 3.5-4.0 kg. The control group included 12 healthy rabbits. Morphometric examination of the cranial part of the optic nerve in both eyes of injured (on the 14-th day after injury) and control animals were used.
Results: Periaxonal degeneration of the optic nerve cranial part was researched. Degeneration was manifested by violation of axon’s flow of axial cylinders that arose as a result of the myelin sheath swelling.
Conclusion: Thus, structural changes of the cranial part of the optic nerve were confirmed with light-ultrastructure studies and morphometric data. These changes come up with the damage of nerve hemodynamic and may serve as a basis for further studies of the pathogenesis and development of strategy of traumatic optic neuropathy treatment in the experiment.
For the first time primary damage to the nervous tissue of the optic nerve caused by partial damage of the central system was described by Garcia-Valenzuela.1 Later secondary degenerative processes that occur in undamaged areas nerve were described,2 which leads to further development of functional disorders.3 The role of compression factors on the formation of secondary degenerative factors and anti- and retrograde parts of the optic nerve in traumatic injuries has not been fully studied. There are several experimental models of such researches. In 2007 Jiang Y4 proposed a model of traumatic damage to the optic nerve canal using surgical tools. Degeneration occurs in a swelling in the affected nerve fibers as a result of compression of nerve tissue. In 2010 Feng Dong-Fu5 suggested to damage the optic nerve by compressing it with aneurisms clips and showed retrograde death of retinal ganglion cells. Thus, the morphological manifestations of lesions of the proximal and distal parts of the ipsilateral and opposite side of visual pathway are still not fully understood and therefore require clarification. The purpose was to clarify ultrastructure features of the optic nerve cranial part damage at traumatic short-time compression of its orbital part in the experiment.
11 sexually mature male rabbits weighing 3.5-4 kg spice Soviet Chinchilla were used as experimental models. The control group consisted of 12 rabbits. The research followed the tenets of the Declaration of Helsinki. Animals were used in the investigation according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The cut skin of right eye was performed in the operation room after a general (Sydazyn 1.5 V/m) and local anesthesia (2% Lidocaine 0.5 ml subcutaneously), antiseptic treatment of the surgical field. The periosteum of outer arch of the orbital process frontal bones was cut and soft tissue was bluntly separated. The optic nerve was detached bluntly. The nerve was taken by surgical clip at the apex of the orbit and behind the muscular funnel. Compression of the optic nerve continued during 10 minutes. The wound was sewn. The main clinical sign of the optic nerve damage was the absence of direct light pupil reaction (Figure 1), that has occurred for 30 minutes and remained abnormal throughout the period of observation (2 weeks).
After 2 weeks the animals were removed from the experiment using guillotine. We examined the morphological cranial part of optical nerves of both eyes. As a control we used appropriate structures of the control group. Examined material was fixed in 2% osmium tetroxide solution for electron microscopic and it was performed and contrasted by generally accepted method. The study was carried out on an electronic material microscope REM 125 K, while accelerating the voltage of 75 kV, magnifications of 1200 to 25,000 times was photographed. Semi-thin sections, thickness of 1 mm, stained with 1% solution of methylene blueprint. Semi-thin sections were examined under a light microscope MS 300 (THR) and photographed using Digital camera for microscope DCM 900 extension 1200-1600. We conducted a morphometric study of electron for an objective assessment of the nerve fibers of the rabbit optic nerve. Negatives were scanned by electron, translated into positive, and stored in the same format. Morphometric calculation was performed on these specimens using software NIH USA “Image J” in manual mode with considering increases.
Measurement for myelin sheaths, axons, and fibers in whole was conducted. Index “g” was determined by the formula: g = a/d, where A - area of the axon, d – area of myelin fibers. The thickness of the myelin sheath was determined by formula: l = D-d, where l - thickness of myelin, D - average diameter of fibers, d - average diameter of the axon. Computer data processing was carried out using the Statistical Package.
Swelling and destructive changes in myelin membranes of nerve fibers (MNF) were observed in the experimental group at light optical level, that led to a decrease in axial cylinders, which in some nerve fibers are not detected, and myelin sheath (MS) of such fibers occupied almost the entire area of the fiber (Figures 2(A&B). We determined some MNF with signs of anisochromy, partial easing and destruction of the myelin sheath. In addition, the small part of the MNF was recorded with preserved structure. Proliferation of connective tissue by newly formed collagen fibers in the endo-and perineural tissue was fixed (Figure 2C).
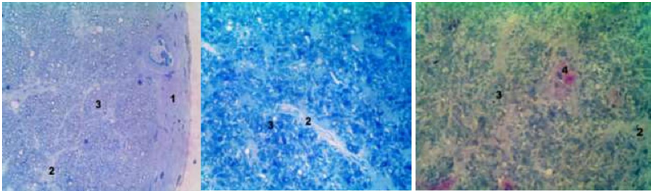
Figure 2 Semi-thin sections of rabbits’ optic nerve cranial part in control (A) and 2 weeks after compression (B,C), stained 1% by Sol. Methylene blue (A, B) and polychrome dyes (C). Inc. x 400. Notes: 1 - reduce axial cylinder nerve fibers, 2 - perineural tissue (4) the newly formed collagen fibers, 3 - the destruction of the myelin sheath.
According to morphometric analysis in experimental group axial area of the cylinder was statistically reduced to 0,73±0,14 mkm2 (it was 1,28±0,34 mkm2 for control group) (p <0.05). A significant growth in the area of MNF to 6,79±1,32 mkm2 (it was 2,73±0,69 mkm2 for control group, (p <0.05)) and increasing the thickness of the myelin sheap <0.05) was noted that evidence about swelling of the latter ones. Index th to 2,11±0,46 mkm (0,53±0,07 mkm in control animals) (G decreased from 0,52±0,03 for control to 0,16±0,05 for injured ones (p <0.05), which also showed edema of Defense, the occurrence of which we associate with a probable reaction of morphological elements Shannov’s cells and in violation of nerve hemodynamic.
Morphological changes were observed in the right optic nerve during electron microscopy (Figure 3A). Axoplasma MS of the fibers was with the signs of edema and it was vacuolated. There were almost no mitochondria, and remained ones were swollen with enlightened matrix, deformed and partially destroyed. There were isolated microtubules and neurofilaments. Strips of myelin were often chaotic, away from each other, becoming wavy course. The above changes are interpreted according to the literature as a violation axonal transport along the axial cylinders,6–8 which is caused by swelling of MS and can be treated as periaxonal degeneration.
Neurolemocyts’ cytoplasm contained many lysosomes, autophagosomes, and myelin degradation products, small and large vacuoles. Their nuclei were fragmented in granular endoplasmic reticulum and Golgi apparatus. There was endoneural swelling, which caused formation of fibroblasts and collagen fibers. Symptoms of demyelination MNF were frequently fixed (Figure 3B). There were specific instances of deep disruption of the myelin sheath and separation of myelin fragments, which were free in the cytoplasm or neurolemmocyte’s axoplasme. Some authors interpret these changes as mesaxonotomy in conditions of impaired energy and protein metabolism.9–11 Metabolic destroys appeared in this neurolemocyte. This converting of cytoplasmic myelin is the base for future reaction of adaptation aimed at ensuring conditions for the regeneration of nerve fibers.
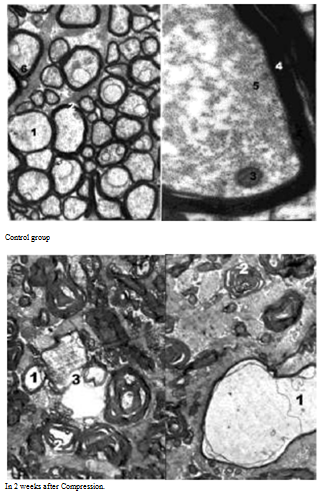
Figure 3 Ultrastructure of rabbits’ optic nerve cranial part. Inc.: A) 12,000 B) 4800. Notes: 1 – axolema’s swelling of nerve fibers, 2 - bundles strips of myelin, 3 - demyelination of nerve fibers.
These changes of MNF were caused by microcirculatory disorders. The most pronounced changes were observed in the capillaries (Figure 4(A&B). The cytoplasm of endothelial cells was homogenized with small and large vacuoles; also there were mitochondria matrix of enlightened and damaged cristae. Nucleuses lost shapes due to significant nuclear invagination envelope. Expansion and partial destruction of structures granular endoplasmic reticulum and the Golgi complex were observed. The luminal surface plasmolemma was formed by enoteliocyte numerous protrusions into the lumen of blood vessels, which subsequently led to destruction of their integrity and formation of microclasmatose. Basement membrane was thickened and layered. There was vacuole degeneration of pericytes. These changes and loss of contact between endotheliocytes caused exit of blood cells and led to the formation of vascular genesis of nerve’s edema. In surrounding connective tissue layer we found an increase in the number of active fibroblasts and collagen fibers. Periaxonal degeneration of the optic nerve cranial part was researched. Degeneration was manifested by violation of axon’s flow of axial cylinders that arose as a result of the myelin sheath swelling.
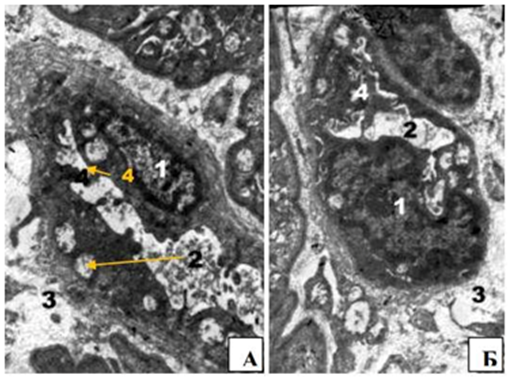
Figure 4 Microcirculatory violation of rabbit’s optic nerve cranial part in 2 weeks after compression. Precapillary (A) and capillary (B). Inc.: x8000. Notes: 1 - The cytoplasm of endothelial cells homogenized with small and large vacuoles (2), mitochondria matrix of enlightened and destroyed cristae core endothelial, 3 – pericapillar edema, 4 -microclasmatos.
Thus, structural changes of the cranial part of the optic nerve were confirmed with light-ultrastructure studies and morphometric data. These changes come up with the damage of nerve hemodynamic and may serve as a basis for further studies of the pathogenesis and development of strategy of traumatic optic neuropathy treatment in the experiment.
ARVO for permitted by IOVS journal policy.
None.
None.

©2016 Nataliya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.