Advances in
eISSN: 2377-4290


Clinical Paper Volume 8 Issue 2
Department of Ophthalmology and Visual Science, Yale University School of Medicine, USA
Correspondence: Brian M DeBroff, Department of Ophthalmology and Visual Science, Yale University School of Medicine, 330 Cedar Street, New Haven, Connecticut, Tel 06520-8061
Received: March 05, 2018 | Published: March 12, 2018
Citation: Broff BMD, Esteban JCR, Servat JJ. UBM measured changes in anterior chamber depth following pediatric IOL surgery with optic capture.Adv Ophthalmol Vis Syst . 2018;8(2):85 –87. DOI: 10.15406/aovs.2018.08.00276
The technique of posterior capture with optic capture was first described by Gimbel and DeBroff in 1994.1 The main principle of this technique involves capturing the IOL optic through a posterior continuous curvilinear capsulorhexis (PCCC) opening leaving the haptics in the capsular bag.1 Many advantages have been described with this technique such as long-term centration of the IOL, maintenance of a clear visual axis2 and the prevention of lens epithelial cell migration responsible for posterior capsular opacities.1 New evidence suggests that the use of acrylic material3 and foldable IOL designs3 can potentially prevent lens epithelial cells migration. Furthermore, the use of anterior vitrectomy has been advocated by some.4‒6 Although the technique of posterior capsulorhexis with optic capture has been used for many years, no previous study has evaluated anterior chamber depths using UBM before or after the procedure. By placing the IOL optic posterior to the posterior capsulorhexis opening an increase in anterior chamber depth may require an adjustment in IOL power calculation. Anterior chamber depth determined by postoperative IOL position can lead to an error in IOL power calculation and has been shown to be one of the principal errors in IOL power calculations.7,8 The purpose of this paper is to study changes in anterior chamber depth (ACD) with Ultrasound Biomicroscopy (UBM) before and after pediatric cataract surgery with the technique of posterior curvilinear capsulorhexis with optic capture and anterior vitrectomy, and compare to the fellow eye in which no surgery or an IOL was positioned in-the-bag without capture.
This study is a prospective evaluation of three patients with developmental cataracts who underwent cataract surgery consisting of posterior curvilinear capsulorhexis with optic capture and anterior vitrectomy. All surgeries were performed by one surgeon: BM DeBroff. An AcrySof acrylic posterior chamber IOL was implanted in each case. Examination under anesthesia (EUA) was performed to obtain keratometry readings, axial length by A-Scan and UBM anterior chamber depth (ACD) measurements. All UBM measurements were performed by the same operator: JC Ramos-Esteban. Measurement of anterior chamber depth defined as the distance between the anterior surface of the lens capsule and the endothelium of the cornea were obtained by the technique described by Pavling.9 Three Ultrasound Biomicroscopy measurements to determine ACD were performed in each eye before and after cataract surgery. The average of three measurements obtained in each eye was used for statistical analysis. The mean and standard deviation was calculated for ACD measurement in each eye. Paired t-test was used to compare ACD measurements.
Three patients (2 females and 1 male), ranging in age from 7 months to 43 months with a mean age of 23.3 months (SD+18.2) were studied. Figure 1 displays the UBM images for each of the 3 cases with arrows depicting measured ACD for each eye both preoperatively and postoperatively. Preoperative axial length measurements and UBM measurements of ACD are listed in Table 1. Case 1 had a change in ACD OD of 0.94 (p=0.0019) from preoperative to postoperative measurements after cataract surgery with posterior caspsulorhexis with optic capture. The left eye underwent cataract surgery with placement of a posterior chamber IOL in the capsular bag without optic capture. The postoperative ACD difference between OD and OS was 1.05 mm (p=0.0010). Case 2 had a change in ACD OD of 1.48 mm (p=0.0003) from preoperative to postoperative measurements. The postoperative ACD difference, after bilateral cataract surgery with posterior capsulorhexis with optic capture, between OD and OS was not statistically significant: 0.23 mm (p=0.1058). Case 3 underwent cataract surgery with posterior chamber IOL implantation with optic capture OS. There was an ACD change OS of 0.29 mm (p=0.0157) from baseline preoperative to postoperative measurements.
|
Mean ACD OD |
Mean ACD OS |
Mean ACD OD |
Mean ACD OS |
A-scan OD |
A-scan OS |
Pre-op |
Pre-op |
Post-op |
Post-op |
(mm) |
(mm) |
|
|
(mm) |
(mm) |
(mm) |
(mm) |
|
|
Case 1 |
2.8 |
2.69 |
3.74 |
N/A |
22.65 |
N/A |
Case 2 |
2.29 |
3.78 |
4.01 |
N/A |
17.65 |
17.65 |
Case 3 |
2.96 |
3.02 |
N/A |
3.31 |
22.76 |
24.19 |
Table 1 Mean UBM (ACD) and A-scan (axial length) measurements
Abbreviations: ACD, Anterior chamber depth; (mm), millimetre
Three patients (2 females and 1 male), ranging in age from 7 months to 43 months with a mean age of 23.3 months (SD+18.2) were studied. Figure 1 displays the UBM images for each of the 3 cases with arrows depicting measured ACD for each eye both preoperatively and postoperatively. Preoperative axial length measurements and UBM measurements of ACD are listed in Table 1. Case 1 had a change in ACD OD of 0.94 (p=0.0019) from preoperative to postoperative measurements after cataract surgery with posterior caspsulorhexis with optic capture. The left eye underwent cataract surgery with placement of a posterior chamber IOL in the capsular bag without optic capture. The postoperative ACD difference between OD and OS was 1.05 mm (p=0.0010). Case 2 had a change in ACD OD of 1.48 mm (p=0.0003) from preoperative to postoperative measurements. The postoperative ACD difference, after bilateral cataract surgery with posterior capsulorhexis with optic capture, between OD and OS was not statistically significant: 0.23 mm (p=0.1058). Case 3 underwent cataract surgery with posterior chamber IOL implantation with optic capture OS. There was an ACD change OS of 0.29 mm (p=0.0157) from baseline preoperative to postoperative measurements.
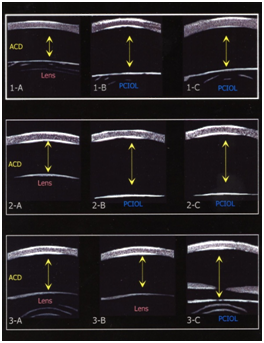
Case 1: A 3 year 7 month old female was referred to the Yale Eye Center for congenital cataract of the right eye. The patient’s birth history was unremarkable. Her past ocular history was significant for congenital cataract OU, status post cataract extraction OS three months prior with IOL placement in the capsular bag (Alcon MA60AC, power 25.0D) by the referring ophthalmologist. Her past medical history includes asthma for which she uses albuterol and an inhaled corticosteroid PRN. Her family history was significant for congenital cataracts and glaucoma in her brother, father, and paternal grandfather. On examination, the lens OD revealed a 5 mm posterior subcapsular opacity, whereas OS had a posterior chamber intraocular lens that was centered and the visual axis was clear. Posterior segment examination was unremarkable. Retinoscopy for the right eye was +0.50 – 0.50 x 180 and for the left eye: +1.00 sphere. Preoperative measurements for OD resulted in average keratometry readings of 42.10 and 45.85 diopters and an axial length of 22.65 mm. Corneal diameters OD were 11.5 mm vertically and 12 mm horizontally and OS were 11.5 vertically and 12 mm horizontally. Average keratometric readings OD were 42.10 and 45.85 and the axial length was 22.65. Intraocular pressure was 22.0 mm Hg OD and 22.5 mm Hg OS. Ultrasound biomicroscopy was performed before the operation OU and 5 minutes after the procedure OD. The patient underwent uncomplicated cataract extraction with primary PCCC, anterior vitrectomy with optic capture through the posterior capsulorhexis with a PC IOL (AcrySof MA60AC, power 24.0 D). By 12 weeks follow-up, the posterior chamber IOL was well centered and the visual axis was clear. Manifest cycloplegic refraction for OD was +1.00 – 1.50 x 30, and OS was –0.50 – 3.50 x 20.
Case 2: A 7 week old female was referred for evaluation of a cataract OS. Her birth history was unremarkable. Family history was negative for congenital or developmental cataracts. On examination, the lens OD had a posterior subcapsular cataract; OS had a dense, opaque white cataract. On EUA, corneal diameters were 11.0 mm horizontally and vertically OU. Average keratometric readings were 49.15 by 53.00 OD and 50.40 and 55.70 OS. Average axial lengths were 17.65 OU. Intraocular pressure was 9 mm Hg OD and 10 mm Hg OS. The patient underwent uncomplicated cataract extraction of the white cortical cataract OS with primary PCCC, anterior vitrectomy with optic capture through the posterior capsulorhexis with a PC IOL (AcrySof MA60AC, power 30.0 D). The postoperative course was unremarkable. At 5 weeks follow-up, manifest refraction OS was +1.75 sphere. At 8 weeks follow-up, the cataract OD was noted to be increased with posterior subcapsular opacification involving the visual axis. EUA was performed and the average keratometric readings were 49.10 by 55.15 OD. Average axial length by A-scan OD was 18.39 mm. intraocular pressures was 10 mm Hg OD and 12 mm Hg OS. The patient underwent uncomplicated cataract extraction, primary PCCC, anterior vitrectomy and PC IOL with optic capture (AcrySof MA60AC, power 30.0 D). At five weeks follow-up, manifest refraction OD was +8.0 D and OS was +9.0 D. The visual axes were noted to be clear OU with no evidence of membranes.
Case 3: A 20 month old male patient referred by his ophthalmologist to the Yale Eye Center for evaluation of a cataract OS. His past medical history was unremarkable. Family history was negative for congenital cataracts. On initial examination, vision OD was fix and follow and OS did not fix or follow with OD covered. The lens in the right eye was clear, the lens OS had a dense posterior subcapsular cataract involving the visual axis. The dilated exam was unremarkable. Average keratometry OS was 40.90 by 42.15D. Average axial length OS was 24.19 mm. Corneal diameters OD were 11 mm vertically and 12 mm horizontally and OS were 11 mm vertically by 11.5 mm horizontally. Average keratometric readings were 41.00 by 41.75 OD and 40.90 by 42.15 OS. Average axial length OD was 22.76 and 24.19 for OS. IOP was 15 mm Hg OD and 14 mm Hg OS. Ultrasound biomicroscopy was performed before the operation OU and five minutes after the operation OS. The patient underwent uncomplicated cataract extraction with primary PCCC, anterior vitrectomy with optic capture (AcrySof MA60AC, power 20.0 D). By 5 weeks follow-up, manifest refraction OD: plano –2.00 x 25, and OS: +0.50 – 1.50 x 165. The posterior chamber IOL was well centered with a clear visual axis.
A significant increase in ACD after pediatric cataract surgery with posterior chamber IOL implantation with optic capture was observed. This significantly increased ACD finding was observed not only when comparing UBM preoperative measurements to postoperative measurements, but also when comparing the one case that had optic capture in one eye and in-the-bag IOL placement in the other eye. By capturing the optic of the IOL posterior to the posterior capsulorhexis, an increased ACD would be expected due to the more posterior placement of the IOL as compared with in-the-bag IOL placement. A constants for particular IOL’s are determined based on an expected final ACD position with the IOL placed in the capsular bag. It is known that decentration and/or placement of an IOL outside the capsular bag can lead to significant spherical equivalent errors.10,11 Because a 0.1 mm change in ACD has been calculated to correspond to a 0.14 D change in spherical equivalent7 our findings of a deeper than anticipated ACD after optic capture are clinically significant for final refractive outcome of pediatric cataract surgery when using the A constant provided by the IOL manufacturer. Past studies have demonstrated sulcus IOL placement to cause a 0.75 mm difference in ACD and recommendation for a 1.0 D reduction in the planned IOL power.12
Our finding of a shift of 1.05 mm between captured and in-the-bag IOL placement along with the findings that eyes with a shorter axial length as typically seen with the pediatric population are associated with higher degrees of refractive error with an ACD shift, 12 point to errors of potentially greater than 2D in final refractive outcome. This posterior shift of the IOL from the intended position would correspond to a hyperopic shift from the predicted value. Although surgeons often aim for a degree of hyperopia postoperatively in the pediatric population to compensate for axial elongation with growth, higher than anticipated hyperopic shifts can be detrimental to planned refractive outcomes. Further studies are needed to evaluate the clinical effect of this deeper than anticipated ACD which may result in surgeon specific A constants for IOL’s that are placed posterior to the posterior capsulorhexis opening. This may help lead to a greater accuracy in predicting post-operative refractions for patients in which posterior capsulorhexis with optic capture is planned.
None.
None.

©2018 Broff, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.