Advances in
eISSN: 2377-4290


Case Report Volume 4 Issue 2
1Department of Ophthalmology, Baskent University School of Medicine, Turkey
2Department of Ophthalmology, Seyfi Demirsoy Hospital, Turkey
3Department of Ophthalmology, Baskent University School of Medicine, Turkey
4Department of Ophthalmology, Izmir University School of Medicine, Turkey
Correspondence: Ali Kal, Baskent University, Department of Ophthalmology, 42080 Konya, Turkey, Tel +090-332-257-0637
Received: March 31, 2016 | Published: April 26, 2016
Citation: Kal A, Kilic BB, Kucukoduk A, et al. Intravitreal bevacizumab injection for choroidal neovascularisation secondary to angioid streaks in pseudoxanthoma elasticum patients. Adv Ophthalmol Vis Syst. 2016;4(2):60-64. DOI: 10.15406/aovs.2016.04.00106
Objective: To evaluate the results of intravitreal bevacizumab (IVB) injections in patients with submacular choroidal neovascularization (CNV) due to angioid streaks (AS) in pseudoxanthoma elasticum (PXE).
Design: Case Series
Population: Six eyes of 4 patients with CNV due to AS were enrolled in this study.
Methods: Subjects were evaluated with visual acuity measurements, direct fundus examinations, fundus fluorescein angiography (FFA) and optical coherence tomography (OCT). Intravitreal bevacizumab 2.5 mg/0.1 ml treatment was applied to the patients who have choroidal neovascularization due to angioid streaks. Follow up examinations were scheduled monthly and injections were repeated when leakage was observed in FFA and subretinal fluid was observed in OCT after first injections.
Results: Mean follow-up time was 7.3 months. During this time enrolled eyes received an average of 3 injections. The mean baseline visual acuity was 0.28 which was improved to 0.43 at the last visit. Mean central retinal thickness was 477.3 at baseline and decreased to 277.5 (range 223-467 μm). No ocular or systemic complications were noted.
Conclusion: Short-term results in 4 patients reveals that the use of IVB for the management of CNV in patients with AS is an effective method. The treatment of CNV secondary to AS with intravitreal bevacizumab provides stabilization in OCT and an improvement or stabilization in VA.
Keywords: pseudoxanthoma elasticum, angioid streaks, choroidal neovascularization, bevacizumab
IVB: Intravitreal Bevacizumab; CNV: Choroidal Neovascularization; AS: Angioid Streaks; PXE: Pseudoxanthoma Elasticum; FFA: Fundus Fluorescein Angiography; OCT: Optical Coherence Tomography; RPE: Retinal Pigment Epithelium; PDT: Photo Dynamic Therapy; VEGF: Vascular Endothelial Growth Factor; AMD: Age-related Macular Degeneration; BCVA: Best Corrected Visual Acuity
Pseudoxanthoma elasticum (PXE) is a hereditary systemic disease of the connective tissue characterized by progressive calcification, fragmentation, and degeneration of elastic fibers in the skin, eye, and cardiovascular system. The gene defect in PXE has been characterized as a loss of function mutation in the adenosine triphosphate-binding cassette subtype C number 6 gene (ABCC6).1 Modification in the extracellular matrix, which is important for the vitality of the retinal pigment epithelium (RPE), appears to be related to this mutation. The most common ocular findings are angioid streaks, which are irregular, characterized with tapering linear breaks in Bruch’s membrane that typically emanate from the optic disc.2,3 Choroidal neovascularization (CNV) is the most serious complication of angioid streaks. Choroidal neovascularization secondary to angioid streaks tends to affect middle-aged patients leading them to a poor visual prognosis when the macular area is involved. Thus, despite the infrequency of angioid streaks, the management of secondary CNV is an issue of concern.4 In addition, visual disturbances occur with development of CNV as a result of retinal pigment epithelial damage or atrophy in the macular area. Finally, fibrous tissue proliferation and retinal pigment epithelial atrophy lead to an irreversible poor visual function. Thus, treatment of CNV is essential to preserve the vision.
Various therapeutic approaches have been tried, but they only delayed the deterioration of visual function. Laser photocoagulation can only halt the progression of CNVs; however it has high rates of recurrences, visual losses, and central scotomas.5,6 Similarly, photodynamic therapy (PDT) also only delays the decline of visual function, though it even increases some CNVs in size.7 Recently, intravitreal administration of bevacizumab, a vascular endothelial growth factor(VEGF) inhibitor that is a full size antibody, which can bind to all isoforms of VEGF-A, has been successfully used as an off-label treatment in CNV secondary to various ocular disorders like age-related macular degeneration (AMD), pathologic myopia, multifocal choroiditis and ocular histoplasmosis.8–10 Up to date, few numbers of studies were evaluated with intravitreal bevacizumab injection in CNV secondary to angioid streaks. In this retrospective study, we aimed to assess the safety and efficacy of intravitreal bevacizumab, in eyes with CNV and angioid streaks.
Patients
Six eyes of 4 patients with CNV due to angioid streaks were enrolled. In all cases, diagnosis of pseudoxanthoma elasticum was depended on skin biopsy. Eyes with any presumed signs of AMD were excluded. The off-label use of the drug and its potential risks were discussed with all patients, and informed consent was obtained from all cases. Patients’s data like age, gender, medical history, ocular symptoms and history, duration of the disease (CNV), prior ocular treatment, interval between the last treatment and the first injection of bevacizumab, and association with PXE (biopsy proven) was recorded. All patients underwent a complete medical examination by an internist to identify potential systemic disease associated with angioid streaks. Detailed ophthalmic evaluation including best corrected visual acuity (BCVA) with standard Snellen chart, slit lamp biomicroscopic examination, fundus photography, fundus fluorescein angiography (FFA) and macular scanning with optical coherence tomography (OCT) was performed prior to the initial injection (Figure 1). Central macular thickness was measured using spectral domain OCT (Optovue RTVue software version 3.5, Optovue Inc., Fremont, CA). Best corrected visual acuity measurements were based on the logarithm of the minimal angle of resolution (logMAR) scale.
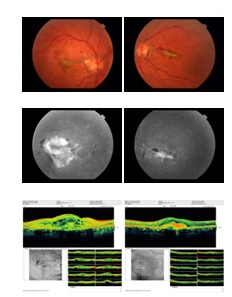
Figure 1 Before anti VEGF injection: Fundus photograph of patient 1 showing choroidal neovascular membrane due to angioid streaks. Fluorescein angiography shows classic subfoveal choroidal neovascular membrane with leakage. OCT shows subretinal edema.
Technique
Injections were performed with topical anesthesia proparacaine hydrochloride under sterile conditions. Periorbital skin was cleaned with povidone iodine 10%. Eyelids were excluded with a speculum, conjunctival sac and the corneal surface was irrigated with povidone iodine 5%. Bevacizumab (2.5 mg/0.1 ml) was injected with a 30-gauge needle into the vitreous cavity from 3.5 mm posterior to the limbus in the superotemporal quadrant. Optic disc pulsatility was checked with indirect ophthalmoscopy, Topical ofloxacin drops were used for 7 days after the injection.
Follow-up
Patients were followed once a month. At each visit, symptoms and BCVA measurements were assessed. Fundus photography and OCT scan were also performed. FFA was repeated only when OCT was not demonstrative. If at least one of the following signs were present, injection was repeated: decreased BCVA with Snellen visual acuity chart, recent macular hemorrhage from CNV, leakage or progression of CNV on FFA, presence of subretinal or intraretinal fluid on OCT or over a 10% increase in macular thickness in comparison with that of the last visit. If signs of CNV recurrence were absent, patient underwent the same procedure after one month.
Six eyes of 4 patients were treated with intravitreal bevacizumab for CNV secondary to angioid streaks. Patient follow-up data are shown in table. There were two men (50%) and two women (50%). The mean age of the patients was 43.6 years (range 34-62). All patients had PXE proved with a skin biopsy. No other systemic diseases associated with angioid streaks were found. The mean follow-up time was 7.3 months (range 3-10 months). All enrolled eyes had classic CNV due to angioid streak. The mean number of injections needed for stabilizing or improving visual acuity was 2.5 (range 1-5). One eye (16.6%) received five injections, one eye (16.6%) received four injections, three eyes (50%) received two injections and one eye (16.6%) received one injection. Initial BCVA of the patients was ranged between 0.05 to 0.8 and the mean VA was 0.28. At the last visit BCVA of patients was ranged between 0.2 to 0.9 and the mean VA was calculated as 0.43. By the last follow-up the increase in BCVA was one line in three eyes (50%), two lines in two eyes (33%) and three lines in one eye (16.6%).
Initial mean central retinal thickness was 477.3 μ (range 776-363 μ) and decreased to 277.5 μ (range 223 – 467 μ) at the last visits. Mean central retinal thickness reduction was 199.8 μ (range 84-541 μ). Complete resolution of intra and/or subretinal fluid on OCT occurred in four eyes (66.8%). In two eyes which two injections were performed (33.2%), intra and/or subretinal decreased but never completely disappeared. Angiographic leakage was completely resolved in six eyes (100%) (Figure 2). No ocular or systemic complications such as endophthalmitis, retinal tear, retinal detachment, uveitis, myocardial infarction or cerebrovascular accidents were noted. Data of the patients are shown in Table 1.
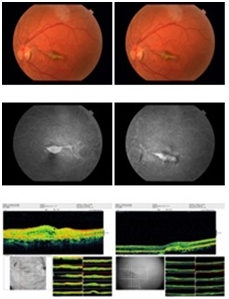
Figure 2 After anti VEGF injection: Fundus photograph of patient 1 showing decrease choroidal neovascular membrane due to angioid streaks. Fluorescein angiography shows decrease in classic subfoveal choroidal neovascular membrane with leakage. OCT shows decrease in subretinal edema.
Patient |
Gender |
Age |
Eye |
VA(Initial) |
VA(Last) |
Follow-up (Months) |
Number of Injections |
CRT (Initial) |
CRT (Last) |
1 |
Female |
38 |
OD |
0.1 |
0.3 |
10 |
5 |
551 |
467 |
1 |
Female |
38 |
OS |
0.05 |
0.2 |
10 |
3 |
383 |
232 |
2 |
Female |
34 |
OD |
0.05 |
0.3 |
6 |
2 |
776 |
235 |
2 |
Female |
34 |
OS |
0.8 |
0.9 |
6 |
1 |
427 |
271 |
3 |
Male |
62 |
OS |
0.4 |
0.5 |
9 |
2 |
363 |
223 |
4 |
Male |
56 |
OS |
0.3 |
0.5 |
4 |
3 |
364 |
237 |
Table 1 Patient follow-up data
OD, right eye; OS, left eye; CRT, central retinal thickness; VA, visual acuity
In this study monthly injection of bevacizumab therapy was found efficient in active CNVs secondary to angioid streaks in patients with PXE. Both decrease in CMT and partial or complete regression of fluorescein leakage were achieved. These functional and anatomic improvements remained stable over the course of the study. Before the era of anti-VEGF, management of angioid streaks associated with CNV had limited success. The efficacy of laser photocoagulation,11–13 transpupillary thermotherapy,14,15 photodynamic therapy (PDT) with verteporfin,16–19 and macular translocation20 for the treatment of CNV was shown to be minor effective methods. In a multicenter prospective trial of PDT in 23 eyes, patients with subfoveal CNV lost 3 ETDRS letters of VA at 12 months.19 In contrast, long-term intravitreal bevacizumab therapy for subfoveal CNV with angioid streaks in 11 eyes, the mean VA improved significantly from 0.28 to 0.56 in 20 months and improved or stable VA was obtained in 88.8% of 9 eyes at 19 months with no significant adverse effects. In the current study with a mean follow-up time of 19 months, the VA in 6 eyes with subfoveal CNV decreased slightly from 0.27 to 0.25 at the final visit, and in 2 eyes, the VA decreased more than 2 lines.21 In our study, a significant decrease in mean central macular thickness was noted (199.8 μm), angiographic leakage diminished or completely resolved in all eyes and intravitreal injection of bevacizumab provided an improvement in BCVA in all eyes. These results were in line with current studies which CNV due to AS was treated with IB and decrease in central macular thickness and stabilization or improvement of VA were achieved.8,10,21–27
The major problem in management of CNV in eyes with AS is the high risk of recurrence. A traditional treatment, laser photocoagulation, resulted in a high recurrence rate of 77%.6 The outcome with PDT is also poor. In spite of the repeating therapies ranging from 2.9 to 3.4 during a mean follow-up of at least 1 year.19 Repeated treatments with laser photocoagulation or PDT can cause ruptures in the Bruch membrane, which is the main cause of failure. Although bevacizumab does not excert direct damage to the Bruch membrane, risk of recurrence seems to be unavoidable in long-term. In early reports on bevacizumab with 6-months of follow-up, Bhatnagar and associates reported recurrence in 1 (11%) of 9 cases, 3 months after the last injection.26 In a recent report of long-term follow-up, complete resolution of subretinal fluid was obtained in 89% of the eyes after 1 or 2 intravitreal bevacizumab injections; however, the mean number of injections was 4.4 during follow-up.10 Thus, managing CNV associated with angioid streaks is complicated and serial bevacizumab injections are needed for CNV to become inactive.
The number of intravitreal bevacizumab injections required to suppress the CNV associated with AS is controversial. Neri and associates reported that the mean interval between bevacizumab injections for CNV due to AS was 4.1 months (range, 1 to 11 months) when patients were monitored by fundus examination and FFA.21 More than half of the eyes in the current case series had recurrent or newly developed CNV in different areas in a minimum 4 months of follow up after the last bevacizumab injection. Based on our study and recurrence rate, new CNVs can develop in different areas after injection of intravitreal bevacizumab and additional bevacizumab injections sooner than 4 months after the previous injection may be necessary to avoid recurrence even after achieving quiescence. In case of CNV recurrence, secondary damage to the surrounding Bruch membrane may not be inevitable; therefore, earlier retreatment should be considered during follow-up. Even small amounts of subretinal or intraretinal fluid in OCT should alert the clinician for recurrence. We did not observe any adverse reactions due to intravitreal bevacizumab injection in our study. In patients with AS secondary to PXE, who have an increased cardiovascular risk profile, potential systemic side-effects of intravitreal bevacizumab such as myocardial infarction or cerebrovascular events need to be assessed prior to injection.28 Longterm effects of repeated injections on retinal and choroidal circulation should also be considered.
Our data seem to confirm the safety and efficacy of intravitreal bevacizumab injection in eyes with CNV secondary to AS in PXE. Currently, no causal therapy for PXE is available. All available treatment options for secondary CNVs have only short-term efficiency, repeated injections are required. With the usage of intravitreal anti-VEGF therapy, patients’s vision may be preserved much longer. Longer-term studies and particularly randomized trials that compare the efficiency of different therapeutic options, would confirm the durability of the visual and anatomical outcome.
None.
None.
The author declares there are no conflicts of interest.

©2016 Kal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.