Advances in
eISSN: 2377-4290


Clinical Report Volume 8 Issue 2
Department of Ophthalmology, Gaziantep University, Turkey
Correspondence: Cüneyt Karaarslan, Department of Ophthalmology, Gaziantep University, Turkey, Tel 90533 731 72 73
Received: March 13, 2018 | Published: April 26, 2018
Citation: Karaarslan C. Importance of immunomodulation in trachoma-related tissue damage and tear function disorders. Adv Ophthalmol Vis Syst.. 2018;8(2):122-126. DOI: 10.15406/aovs.2018.08.00284
To put forward better prevention and treatment strategies in trachomatous tissue damage and tear function disorders, a field survey was performed in a village in the province of Turkey, which is a settlement with endemic trachoma according to the World Health Organization. Samples of tears, upper tarsal conjunctiva, and serum were taken from patients with 5 different stages of trachoma, including 13 patients in the initial stage and 15 in each of the other stages. Samples were also collected from 15 healthy control patients. We measured the levels of interleukin (IL)-1, IL-2, epidermal growth factor (EGF), and tumor necrosis factor (TNF) using enzyme-linked immunosorbent assays (ELISAs). We measured immunoglobulin M (IgM), IgA, and IgG levels in tears using immunodiffussion plates. EGF, human leukocyte antigen – antigen D related (HLA-DR), and interleukin -2 receptor (IL-2R) expression, and lymphocyte and monocyte infiltration were examine d using direct immunofluorescence of upper tarsal conjunctiva samples. Antichlamydial IgM, IgA, and IgG serum levels were also measured. The distribution of trachoma in this residential area and its complications according to age and genders were determine d using additional field scans. Data analysis revealed that the significant increase in the levels of neutrophils, IL -1, TNF, and cytotoxic T lymphocytes, which are the indicators of nonspecific immune response in the early stages of trachoma, results in suppression/dominance of IL-2, IL-2R, HLA-DR, helper T cells, and 13 lymphocytes and immunoglobulins as the disease progresses. EGF increases to very high levels in later stages.
Keywords: trachoma, chlamydia trachomatis, immune response, tissue damage
Trachoma is a chronic contagious eye disease caused by Chlamydia trachomatis, which can cause repeated episodes of usually bilateral, often active, conjunctivitis followed by scarring in children and young adults. The scarring can result in vision loss later in life.1–3 Although the global incidence and severity of trachoma have dramatically decreased in the last 30 years, it is still among the most common causes of preventable blindness in North Africa, the Middle East, and parts of Asia.4–6
Agent of infection
The infectious agent, Chlamydia trachomatis, is an intracellular parasite. It can be find in two different form in the cells. ı.Elemental bodies; are infectious bodies with an equal ratio of RNA to DNA that are surrounded by a three layer wall with a nucleus and ribosomes and their diameter is 0.2-0.4 micron. ıı.Reticular (initial) bodies; are non-infectious bodies capable of reproduction with intense RNA and their diameter is 0.8-1.5 micron.7
Epidemiology
Trachoma often occurs at the age of 0-6 years; infants 2-3 months of age are susceptible in hyper-endemic settlements. According to the WHO such areas have poor hygiene and inadequate sanitation.8 Transmission is closely related to environmental factors such as dusty, hot, dry environments. The Musca domestica and Musca sorbens species of flies are also very important vectors in endemic areas because they feed on lacrimal secretions.9,10
Classification
In 1987, the WHO proposed an easier and more comprehensive classification than the complex scores in use at the time Trachomatous inflammation may be classified as follicular or intense. In cases of trachomatous inflammation - follicular (TF), the upper tarsal conjunctiva has 5 or more follicles with a diameter of at least 0.5 mm that are white, gray, or yellow in color. In cases of trachomatous inflammation - intense (TI), the tarsal conjunctiva is thickened and more than half of the deep tarsal veins normally visible are covered. TI may be further classified by additional symptoms. In cases with trachomatous scarring (TS), the tarsal conjunctiva also has bright, fibrous scar tissue in the form of a white line, tape, or layer. In cases of trachomatous trichiasis (TT), at least one eyelash is in contact with the eye. In cases of corneal opacity (CO), there is opacification in the region that fits the pupil area of the cornea that reduces visual quality.11
Clinical diagnosis
A total of 88 cases of trachoma in 6 groups were included in this study. The control group included 15 healthy cases We also included 13 patients newly infected with trachoma (starting phase), 15 patients with mild active inflammatory infection who were in the follicular period (TF group), 15 patients with severe active inflammation (infection and pathogenic trachoma (TI)group), 15 patients who had trachoma with scarring (TS group, active inflammation had disappeared), and 15 patients with trachomatous trichiasis, in which inflammation had nearly disappeared (TT group).
Laboratory findings
The following laboratory methods can be used to diagnose trachoma. Upper tarsal conjunctiva scrapes or biopsy specimens may be tested using Giemsa staining or direct fluorescence antibody examination. In Giemsa staining, inclusions in the cytoplasm of blue-stained epithelial cells near the nucleus that appear red during the elementary period and blue in reticulocytes are diagnostic. These bodies are very difficult to detect, especially in the inactive period, so the sensitivity of the test is very low. Direct fluorescence antibody examination is based on the use of previously acquired fluorescein -marked antibodies against the species-specific chlamydial major outer membrane protein (MOMP) antigen. In addition, this method can be performed using serotyping. There are 15 serotypes of Chlamydia trachomatis. Serotypes A, B, and C are the agents of trachoma, serotypes D-K cause oculogenital infections, and serotypes L1, -2, and -3 cause lymphogranuloma venereum.12,13
Treatment
The treatment of trachoma begins with the correction of hygienic and socioeconomical conditions, The recommended medical treatment time is for the control of active inflammation or the eradication of the pathogen is in the inactive period in which complications may be present. Some complications of trachoma (e.g. trichiasis, entropion, etc.) may require surgical intervention. According to the WHO, topical medical treatment (sodium sulfacetamide, erythromycin, or tetracycline drops) in the follicular period of cases with very severe inflammation should be used in conjunction with systemic treatment (sulfathiazole, doxycycline.14
Immunology
By using enzyme-linked immunosorbent assays (ELISAs), polymerase chain reaction (PCR), or molecular hybridization, chlamydial antigen or anti-chlamydial antibody can be detected in tears and serum samples. The specificity is 97 -98% and the sensitivity is 85-97%. in PCR, chlamydial DNA can be amplified from any sample that contains the organ ism. It is less time-consuming than other methods (results can be obtained in 4 hours), more sensitive, more reliable, and easier. Molecular hybridization of ribosomal RNAs from Chlamydia trachomatis (molecular rRNA hybridization screening) is also a less time-consuming and reliable method; screening for chlamydial rRNA using molecular hybridization is preferred for early diagnosis.15
The village in the province of Kilis, Turkey, a settlement susceptible to the epidemic spread of trachoma according to the WHO, was selected. In August 1993, conditions were suitable for an epidemic. 258 individuals were examined and 118 of these people had different stages of clinical trachoma. A control group was formed by including 15 healthy individuals who had no symptoms or complaints suggestive of trachoma. Patients with trachoma were first divided into 5 groups according to stage and 15 patients at each stage were randomly selected.
Upper tarsal conjunctival biopsy
Drop anesthesia was applied and a few minutes later, the upper lid was reversed and a tissue incision 1.5 mm in diameter was collected from the upper fornix conjunctiva region using a fine needle or a conjunctiva blade. The samples were stored at -20°C in accordance with the frozen section method and were transferred to the laboratory on dry ice molds in sealed boxes. Tissue samples were then collected from the embedded columns as sections 4 micron thick and were marked with fluorescein and examined using fluorescein microscopy. The staining properties were evaluated by the same person who evaluated the preparations; the samples were classified with (-) and (+) marks according to the intensity of fluorescence.
Tear samples: The nasal mucosa was stimulated to tear release reflex then tears were collected from lower conjunctival sac using a micropipette.
Serum samples: 3 mL blood sample was collected from the median cubital vein. After centrifugation the serum was collected.
We detected trachoma in various stages in 118 of 258 cases (prevalence, 45.73%) examined in that relevant settlement.52 of 118 (44%) trachoma patients were male; 66 (56%) were female. The average age was 27.3 years (range, 3 months - 82 years). As shown in Table 1, most patients with trachoma (56%) were in the inactive phase and were >25 years old. The distribution and percentages of the patients according to complication are shown in Table 2. The most common eyelid complications observed in this study were trichiasis, entropion, ectropion, and ptosis. The distribution and percentages of conjunctival complications are shown in Table 3. The most common conjunctival complication observed in this study was the Arlt’s line, followed by symblepharon and xerosis. Table 4 shows the distribution and percentages of corneal complications.
Age(years) |
Stage |
Number of cases (%) |
Sex (male/female) |
0-6 |
Starting-TF |
16 13.7 |
7/9 |
Jul-15 |
TF |
19 16.1 |
6/13 |
16-24 |
TF-TI |
17 14.2 |
7/10 |
25-40 |
TS-TT |
31 27.8 |
13/18 |
>41 |
TT-CO |
35 28.2 |
19/16 |
TOTAL |
|
118 100 |
52/66 |
Table 1 Distribution of the mean age groups, sex, and clinical stages of trachoma cases
Abbreviations: TF, follicular trachoma; TS, trachomatous scarring; TI, intensive trachoma; TT, trachomatous trichiasis; CO, corneal opacification.
Complication |
Number of eyes (n=118) |
% |
Trichiasis |
14 |
11.86 |
Eyelid distortion |
9 |
7.62 |
Entropion |
8 |
6.77 |
Ectropion |
2 |
1.69 |
Ptosis |
l |
0.84 |
TOTAL |
34 |
28.78 |
Table 2 Eyelid complications of trachoma
Complication |
Number of eyes (n=118) |
% |
Arlt’s line |
24 |
20.33 |
Symblepharon |
5 |
4.23 |
Xerosis |
3 |
2.54 |
TOTAL |
32 |
27.1 |
Table 3 Conjunctival complications of trachoma
Complication |
Number of eyes (n=118) |
% |
Pannus |
74 |
62.7 |
Herbert pits |
66 |
55.93 |
Corneal opacity |
9 |
7.62 |
Salzman's nodular degeneration |
5 |
4.23 |
Epithelial keratopathy |
3 |
2.54 |
Corneal ulcer |
1 |
0.84 |
Subepithelial keratopathy |
0 |
0 |
Table 4 Corneal complications of trachoma
The most common corneal complications observed in this study were pannus and Herbert pits, followed by corneal opacity, Salzman's nodular degeneration, epithelial keratopathy, and corneal ulcer. None of patients in this study had sub -epithelial keratopathy. EGF, TNF, and IL-1 and IL-2 levels in the tears of trachoma patients and 15 healthy eyes are shown in Table 5. The increase in IL-1 in TF, and TI stages of trachoma was significant compared to the control group and the TS -TT-CO groups (p=0.01). The changes in IL-2 concentration was not significant between the control group and the other groups (p>0.05). The increase in TNF in patients in the TF and TI stages was significant compared to the control group but was not significant between the TI and TS or the TT-CO groups (p=0.01, p>0.05). When compared to the control group and other groups, the increase in EGF in the TS and TT-CO groups was significant (p=0.01, p˃0.05).
Stage |
IL-1 (mg/mL) |
TNF (ng/mL) |
IL-2 (ng/mL) |
EGF (ng/mL) |
Control |
1.02 |
57.2 |
72 |
0.018 |
I |
29.28 |
232.4 |
94 |
0.023 |
II |
16.7 |
169.5 |
154 |
0.022 |
III |
l.93 |
98.5 |
104 |
0.825 |
IV |
1.03 |
88 |
69 |
1.612 |
Table 5 Immununomediator levels in tears of control and trachomatous eyes
IL-1, interleukin 1; TNF, tumor necrosis factor; IL-2, interleukin-2; EGF, epidermal growth factor; C, control group; I, starting trachoma; stage II, follicular or intense trachoma; stage III, trachomatous scarring; stage IV, trachomatous trichiasis and corneal opacity.
Immunofluorescence characteristics of the conjunctival biopsies of 15 eyes within the control group and 73 trachomatous eyes at different stages were evaluated by allocating ‘’-‘’ and “+” marks according to fluorescence intensity and density in Table 6. As seen in Table 7 increasing of IgM level in the acute phase of trachoma was not statistically significant compared to the control group (p>0.05). There was no significant difference in IgG values among groups (p>0.05). Although the high concentrations of IgA observed in stages I, II, and III were not significant, they indicate that that the humoral immune response that develops in tears is primarily due to IgA. In addition, though the decrease of IgA in tear concentrations at stage IV was remarkable, it was not significant (p>0.05). The incidence and emergence of serum specific antichlamydial antibodies as the percentage of cases of trachoma are included in Table 8.
Stage |
CD2 |
CD |
CD8 |
CD14 |
CD10 |
IL-2R |
HLA-DR |
EGFr |
C |
+/- |
- |
- |
+/- |
- |
- |
- |
t |
- |
||||||||
++ |
||||||||
+ |
||||||||
- |
||||||||
I |
+++ |
+ |
+++ |
+ |
- |
+ |
+ |
|
II |
+++ |
+++ |
++ |
++ |
++ |
++ |
+ |
|
III |
+++ |
++ |
++ |
++ |
+ |
+++ |
++ |
|
IV |
|
++ - |
+/- |
+/- |
|
- |
++ |
+++ |
Table 6 Conjunctival biopsy findings of control and t rachoma cases
CD2, all T lymphocytes; CD4, helper T lymphocytes: CD8, cytotoxic/ suppressive T lymphocytes; CD14, monocytes; macrophages, and granulocytes; CD19, B lymphocytes; IL-2R, activated T (predominant) and B lymphocytes; HLA-DR (human leukocyte antigen- antigen D related), cells displaying the antigen; EGFr, epidermal growth factor receptor.
Stage |
IgM (mg/ml) |
IgG (mg/ml) |
IgA (mg/ml) |
C |
0.21 |
0.32 |
1.14 |
I |
0.23 |
0.27 |
1.87 |
II |
0.18 |
0.41 |
1.92 |
III |
0.19 |
0.28 |
1.79 |
IV |
0.14 |
0.23 |
0.81 |
Table 7 Tear immunoglobulin levels in control and trachomatous cases
Percentage (%) of cases |
Stage I |
Stage II |
Stage III |
Stage IV |
80-60 |
IgM |
|||
60-40 |
IgG |
IgA |
||
40-20 |
IgA |
IgG |
||
IgM |
IgA |
|||
10-0 |
|
|
|
|
Table 8 Emergence of serum-specific antichlamydial antibodies as the percentage of cases of trachoma
Although the immune response to trachoma has attracted interest among researchers, sufficient effective research on this topic has not been performed on humans. This study has revealed the following: Chlamydia trachomatis first activates a cellular immune response by infecting the conjunctival and corneal epithelium. In this period, the intense infiltration of monocytes, macrophages, polymorphonuclear leukocytes, and T lymphocytes (CD2), especially T suppressor (CD8) cells, develops under the epithelial region of the upper tarsal conjunctiva. The infectious agent is presented to effect or cytotoxic cells within the major histocompatibility complex (MHC) class I system. There is a simultaneous increase in the tear concentrations of IL-1 and TNF released from activated monocytes and T lymphocytes, while there are small changes in the levels of IL-2 and EGF.
IL-1 activation induces the flow of T helper (CD4) and B lymphocytes (CD19) to the subepithelial region, especially to the lymphoid islets, which activates the humoral immune system. Activated T cells increase the HLA -DR expression in the deeper tissues of conjunctival epithelial cells, and some preparations present chlamydial antigens via MHC Class -II complexes (Figure 1). In this period antichlamydial antibodies can be detected in tears, while the levels of IL-1 and TNF in tears decrease, the level of IL-2 increases, and intense IL-2R staining in tissue samples increases. Intense polymorphonuclear leukocyte infiltration during the onset of infection has a significant chlamydial effect, although the polymorphonuclear leukocytes are later replaced by mononuclear cells. In later stages, it was observed that the level of IL-2R (+) was decreased while T lymphocyte suppression continued, and HLA-DR (+) was vigorously continued in tissue samples. The most striking change observed in this period was the increase in both the staining intensity and the staining density of EGF, which were lined along the entire epithelium (Figure 2).
As the levels of antichlamydial IgG and IgA in tears were maintained, IL-1-2 and TNF in tears were decreased, the significant increase in the EGF level detected by staining agreed with the tissue findings. Trachoma develops as an autoimmune disease rather than an infectious disease. The increasing immune effect of EGF, which kills self-alienated epithelial cells that express HLA-DR, forms scar tissue due to fibroblast activity. The "shrinkage" feature of collagen and elastin fibrils, which increase in the environment due to intense fibroblastic activity, leads to decreases in size and irregularities in small areas of conjunctival tissue (Figure 3) (Figure 4).
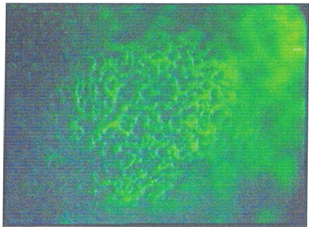
Figure 1 Human leukocyte antigen–antigen D related (HLA-DR) antigen expression using fluorescein isothiocyanate (FITC)-stained monoclonal antibodies in deeper tissues of a conjunctival biopsy of a 17 -year-old trachoma patient in the inflammation stage of the disease (×l00).
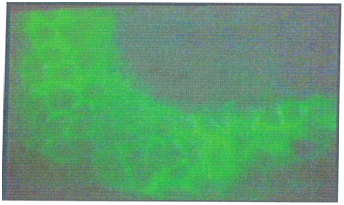
Figure 2 EGF receptors throughout the epithelium, visualized using fluorescein isothiocyanate (FITC)–stained monoclonal antibodies in a conjunctival biopsy of a 32-year-old trachoma patient in stage III (trachomatous scarring) (×100).
Trachoma becomes an immune disease rather than an infectious disease after a certain point. Thus, determination of the problem and creation of a timeline of development can aid the prevention, prophylaxis, and treatment of complications leading to loss of vision.
None.
The author declares that there is no conflict of interest.

©2018 Karaarslan. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.