Advances in
eISSN: 2377-4290


Research Article Volume 3 Issue 2
1Department of Neuropsychiatry and Behavioral Science, Federal University of Pernambuco UFPE, Brazil
2Department of Psychology, Federal University of Pernambuco UFPE, Brazil
3Department of Psychology Cognitive Neuroscience and Behavior, Federal University of Para ba UFPB, Brazil
Correspondence: Jakina Guimaraes Vieira Gutemberg,Department of Neuropsychiatry and Behavioral Science,Laborat rio de Percep o Visual Lab Vis UFPE, Federal University of Pernambuco UFPE, Recife Pernambuco Brazil Rua Acad mico H lio Ramos S N Cidade Universit ria CEP 52071 000 Recife Pernambuco, Brazil, Tel (83) 98862-4441
Received: August 27, 2015 | Published: November 13, 2015
Citation: Guimaraes VGJ, Bustamante SML, Antonio SN. Importance of contrast sensitivity to high and low spatial frequency and artificial neural networks for visual impairment in multiple sclerosis. Adv Ophthalmol Vis Syst. 2015;3(2):272-278. DOI: 10.15406/aovs.2015.03.00084
This research compared contrast sensitivity (CS) to high and low spatial frequency of angular frequency stimuli and sine-wave gratings, respectively, to assess adults diagnosed with multiple sclerosis (MS), MS with optic neuritis (ON) (MSON) and control. We also investigated whether the involved cognitive impairments affect CS, and whether the technique of artificial neural network (ANN), coupled with the CS to only two spatial frequencies, correctly predict MS. The present cross-sectional study measured CS of adults MS, MSON and control, 22-50 years-old, that used a psychophysical procedure designed with the forced-choice method. The t test for independent samples showed that the CS was better in the control, followed by MS and MSON. The MSON group had lower CS values at all tested angular frequency stimuli and low sine-wave gratings as well (p<0.0001). The frequency 24 cycles/360° and 4.0 cpd were the ones that best differentiated the groups surveyed. The neuropsychological test stroop was the only one to yield a difference between groups (p<0.0001). There was no correlation between the psychophysical measures and the protocols of optical coherence tomography. These data suggest that visual impairments found in MS and MSON are related to CS. The ANN 44221 architecture was successful in separating people with MS from controls, by using the spatial frequencies of 24 cycles/360º and 4.0 cpd that showed good correlation performance (0.71) in the test phase. Research employing this perspective will proceed with the main purpose of verifying the extent to which the use of CS for sine-wave gratings and angular frequency stimuli are relevant in the early assessment of MS.
Keywords: multiple sclerosis, contrast sensitivity, artificial neural network, visual impairment, angular frequency stimuli
CS, contrast sensitivity; MS, multiple sclerosis; ANN, artificial neural network; CNS, central nervous system; ON, optic neuritis; OCT, optical coherence tomography; VA, visual acuity; CSF, contrast sensitivity function; CG, control group; ONH, optic nerve head; GCC, ganglion cell complex; FLAIR, fluid acquisition inversion recovery; RAVLT, rey auditory-verbal learning test; SWI, susceptibility-weighted imaging
Multiple sclerosis (MS) is a complex neurological disorder of the central nervous system (CNS), with characteristics of an autoimmune, demyelinating, inflammatory, and degenerative disease1–6 Visual dysfunctions7,8 are the most frequent clinical findings of this disease; and research by Villoslada et al.9 lists many central nervous system impairments, particularly on the retina, optic nerve, chiasm, optic tract, lateral geniculate nucleus, optic radiations, primary visual cortex, and visual ancillary areas. Optic neuritis (ON) may be an early symptom associated to MS in up to 38% of the MS population. Under this condition, there may be difficulties in detecting color saturation, mainly after acute ON.10 Findings by Reis et al.11 support this observation, and go further in supporting that there may be changes in the color discrimination, even in cases of MS without ON. Optical coherence tomography (OCT) is a noninvasive neuroimaging technique that sampless the anterior visual pathway, and, by implication, the CNS, and can quantify the level of retinal and optic disc atrophy caused by axonal degeneration9,12–20 The loss of contrast sensitivity (CS) in MS, even when that population has preserved visual acuity (VA) of 20/20, is well established. The findings of Graves et al.21 show important contributions in this context, by identifying improvement of CS in MS through neuroprotective therapies with Alemtuzumab. Yet, there is no unanimity on CS measurement protocols.
Among the methods most often used are the cards of Pelli-Robson19,7,8,10,22,23 those of Sloan, and CSV-1000E.24 Regan et al.25 were the first to report subclinical visual changes in MS resulting from impairment in CS, as estimated by the measurement of the contrast sensitivity Function (CSF) using sine-wave gratings. Vieira-Gutemberg et al.26 replicated the findings of Regan et al.25 with sine-wave gratings, and were the first to measure CS in people with MS, and without a history of ON, using angular frequency stimuli. Angular frequency stimuli are defined in polar coordinates and is known in the literature as radial grid, polar grid, or windmill. But it was first defined as angular frequency stimuli by Simas27 Simas and Dodwell.28
It was established by these authors that the human visual system is more sensitive to angular frequency stimuli than to sine-wave gratings. This is probably due to the fact that its configuration stimulates neurons in various orientations Vieira-Gutemberg et al.26 observed a loss in CS in people with MS. It occurred at the maximum sensitivity range of the angular CSF, that is, frequency of 24 cycles/360º. In this same research, CS to sine-wave gratings was measured with a computerized protocol29 that allowed manipulation of contrast characteristics, stimulus orientation, luminance, and the choice of the spatial frequency in real time.
Despite the importance of measuring CS in people with MS using spatial frequency stimuli, the procedures are time consuming in the clinical setting, since at least four stimuli are necessary, and the measurement has to be monocular. Attempts to speed up the investigation of MS from neuroimaging, such as magnetic resonance imaging, made of artificial neural network (ANN) a technology that is now widely used in clinical research.30 The ANN is a mathematical model that approximates functions31 and seeks to emulate brain operation, acting as a parallel processor that stores knowledge and makes it available for use.
The design of an ANN initially depends on the definition of architecture. The first phase of this project is learning (training); and, the second, the test. In this one, the ANN recognizes new data from the knowledge gained in the training phase. In the test phase, occurs a generalization that, according to Garcia-Martin et al.2 would be the property of the ANN to produce the appropriate output for data that were not present during training.
The way the neurons are organized in the architecture of an ANN depends on the learning algorithm used to train it. The learning by error correction emerged as the most widespread learning algorithm to train an ANN of multilayer perceptron (MLP) type. In its wider definition, MLP is a feed forward ANN model that maps sets of input data onto a set of appropriate output. The MLP is a type of ANN feed forward, consisting of an input layer, one or more hidden layers, and one for output. The neurons of the hidden layer enable the ANN to learn complex tasks, extracting the most significant characteristics of the input patterns. The MLP has been used to solve problems with supervised training by error back propagation algorithm, which is based on the learning rule for error correction. This rule decreases the difference between the desired response, and the actual response of the specific ANN. This difference is defined as error signal (ɛ), adjusted step by step during the training phase of the neural network. To better understand: the output signal (yk) of an ANN of a given neuron k is compared with a desired response represented by dk. Then, the error is represented as follows: ɛk=dk - yk.
Research shows that cognition is often compromised in MS, affecting up to 65% of this population.32 The cognitive areas most affected in MS are attention, working memory, executive functions, and inhibitory control.33–38 The present study will use neuropsychological tests to verify cognitive impairment and possible relations with the affected CS. Other objectives are: (i) to evaluate CS of adults with MS, with MS and history of ON (MSON), and a control group (CG), using angular frequency stimuli and sine-wave gratings. Further, this research intends to investigate whether the use of ANN technology is able to correctly classify people with MS or control from the input of CS using a reduced number of spatial frequencies, two if possible.
This study was submitted to the Ethics and Research Committee of the Health Sciences Center of Federal University of Paraíba, João Pessoa, Paraíba, Brazil, under the protocol number 0529/13. Presentation Certificate for Ethical Appreciation: 20171613.4.0000.5188. Participation was voluntary. Data were collected only after the signing of the informed consent term.
Participants
Participants were 24 volunteers (22-50 years old), 12 of them with clinically defined MS in the clinical forms relapsing-remitting or RR (10), and secondary progressive or SP (2), according to McDonald’s criteria. Of the total participants with MS (10 female and two male, with a mean age of 39±7.24 of standard deviation), eight had no history of ON, and four had ON. All patients were followed by a neurologist specialized in the diagnosis of MS, and by two neuro-ophthalmologists (Table 1). The remaining 12 volunteers were in the CG and in good health (seven females and five males, with mean age of 33±9.63 of standard deviation).
Participants |
Sex |
Age |
Time of disease from diagnosis |
Location of lesions |
1 |
F |
47 |
2 Years |
Lesions in the periventricular white matter of the cerebral hemispheres, inferior frontal gyrus, left cerebellar peduncle left. |
2 |
F |
38 |
3 Years |
Lesions in the periventricular white matter of the cerebral hemispheres, coronae radiatae, semioval center, junction calososeptal bridge, cerebellar peduncle top left. |
3 |
F |
39 |
3.2 Years |
Lesions in the periventricular white matter and subcortical of cerebral hemispheres, coronae radiatae, semioval center right and bridge to the right. Lesions between C2 and C6. |
4 |
F |
43 |
6.1 Year |
Lesions in the periventricular white matter of the cerebral hemispheres and subcortical supratentorial, junction calososeptal and infratentoriais. |
5 |
M |
37 |
12.4 Year |
Lesions in the periventricular white matter of the cerebral hemispheres, semioval center and white matter peritrigonal. |
6 |
F |
25 |
2 years |
Lesions in the periventricular white matter of the cerebral hemispheres and pericalosais of cerebral hemispheres intramedullary juxtacortical lesion in frontal gyrus top left. White matter of the spinal cord in cervical segment. |
7 |
F |
37 |
7 years |
Lesions in the periventricular white matter of the cerebral hemispheres, coronae radiatae, semioval center and junction calososeptal. |
8 |
M |
50 |
11 Years |
Lesions in the white matter of the spinal cord between C7-T1 and between T11-T12. |
9 |
F |
36 |
14 Years |
Lesions in the periventricular white matter of the cerebral lobes, semioval center and junction calososeptal. |
10 |
F |
33 |
6 Years |
Lesions in the periventricular white matter of the cerebral hemispheres, semioval center and junction calososeptal. |
11 |
F |
49 |
6 Years |
Lesions in the periventricular white matter of the cerebral hemispheres, junction calososeptal, semioval center right and deep areas of the left parietal lobe. |
12 |
F |
34 |
10 Years |
Lesions in the periventricular white matter deep and subcortical bilaterally, especially, junction calososeptal. Increase in the size of lesions in subcortical region (frontal, parietal and insular) left. |
Table 1 Clinical characteristics of participants with multiple sclerosis
Criteria for inclusion and exclusion
In the city of João Pessoa, Paraíba, Brazil, we randomly selected adults 20-50 years old, with time available to participate in the research. In the particular case of adults with MS, it was necessary to undergo a clinical, neurological and neuro-ophthalmological examination, all within the last six months. Also, they could not be in a neurological outbreak for the same period. Volunteers - with and without MS - should have satisfactory performance in color vision tests with the blades of Ishihara, and with plastic, circular sheets (cards) covered with Munsell’s paper. This test is called the color arrangement, or Lanthony Desaturated (D-15d) test. In addition, these volunteers should have monocular VA of at least 20/20, normal or corrected to normal vision, as assessed by Rasquin’s chart of optotypes “E”, at high contrast, at a distance of 400 cm. Volunteers that were experiencing peripheral neuropathy, cataract, amblyopia, strabismus, glaucoma, astigmatism not corrected, dyschromatopsia, optic neuropathy [19], VA 20/20, arterial hypertension, stroke history, migraine, not reactive depression, panic syndrome, smoking, and alcoholism did not participate in the study.
Equipments
A 19-inch LG CRT (Cathodic Ray Tube) color monitor connected to a BITS ++ (Cambridge Research Systems, Rochester, United Kingdom) unit through a DVI connector was used to produce the stimuli. The linear, dynamic contrast was converted into 256 gray levels for each display color. The screen luminance was calibrated (Long To, 2013), with gamma correction of 64 points, and measured with a Color CAL photometer (Cambridge Research Systems). The red, green, and blue color of the monitor screen were adjusted with maximum luminance of 31.11 cd/m2 (candela per meter squared), 80.97 cd/m2 and 16.31 cd/m2, respectively. A table equipped with a forehead and chin rest together with a mouse was used as well.
Visual stimuli
The angular frequency stimuli and sine-wave gratings, achromatic, were presented through a circular static window. The spatial frequencies of the two classes of stimuli were calibrated for a distance of 300 cm from the monitor screen center to the eyes of the observer. The tested spatial frequencies were 1, 3, 4, 24, and 48 cycles/360º and 0.5, 1.0, 3.0, 4.0 and 7.5 cycles per degree of visual angle (cpd) for sine-wave gratings. The initial default contrasts-a suprathreshold stimulus, “of sufficient strength or quantity to produce a perceptible physiological effect used for each spatial frequency were: 1 (0.071); 3 (0.063); 4 (0.055); 24 (0.024), and 48 (0.024); and 0.5 (0.079); 1 (0.087); 3 (0.094); 4 (0.094) and 7.5 (0.102), respectively. The stimuli were generated in photopic condition, with mean luminance of 41.05 cd/m2; and the room illuminance was set to 286 lux.
Procedures
We used he psychophysical method of forced-choice 39 that is based on the probability of correctly perceiving the stimulus. In this method, the participant has to choose the temporal position of the test spatial frequency as compared to a uniform field of mean luminance (noise) of 41.05 cd/m2. Prior to the session, volunteers were instructed to press the left button of the mouse when they perceived the test stimulus (angular frequency stimuli or sine-wave gratings) in the first temporal position; and the right button otherwise - that is, after the uniform field at mean luminance. Thus, the task of the volunteer was correctly choosing the stimulus containing the test spatial frequency pattern (angular frequency stimuli or sine-wave grating).
During each experimental session, a sequence of pairs of stimuli was presented. The session began with a beep, immediately followed by the presentation of the first stimulus (test stimulus or uniform field) for 2 seconds. After a 1-second interval, the next stimulus (test stimulus or uniform field) also appeared for 2 seconds. The order of presentation was randomized and controlled by a program C ++. If the volunteer response was correct, it would be followed by another beep and a 3-second interval for the sequence to be repeated. The interval between trials was of at least 3 seconds, regardless of the answer is correct or not. All participants with MS, MSON, and CG went through all conditions, on different days. Estimates of CS were recorded with monocular vision under photopic conditions and mean luminance set at 41.05 cd/m2. Counterbalances between eyes, class of stimuli, and spatial frequencies were performed.
Lanthony desaturated test
The test protocol D15d40 was applied binocularly. The test is performed using 15 circular chips in the Munsell color space. The participant had unlimited time to put the chips in the correct order of saturation. The data were analyzed according to the procedure fixed,41 that provides the total color distance score as indicative of sensitivity; and with the color confusion index (CCI).9 The volunteer who made no errors in the ordering receives a CCI value of 1.0.
Neuropsychological tests
Assessment of cognitive functions such as attention, memory, and executive functions of adults - with and without MS - was performed by a single researcher using the following neuropsychological tests, in this order: direct digits (sustained attention) and reverse (working memory); test of the codes and search for symbols, both for analysis of selective attention; stroop test (inhibition of responses) and Rey auditory-verbal learning test (RAVLT) (Rey, 1964).
Optical coherence tomography
The retina and the optic nerve head of all participants were evaluated by OCT from Optovue (Fremont, California, United States of America), using the Ivue software, version 1.4. Two protocols were used: optic nerve head (ONH), and ganglion cell complex (GCC).
Magnetic resonance imaging
The registration protocol of the location of demyelinating lesions in adults with MS was done with gadolinium enhancement on T2 magnetizing time, and by the weighted technique at time T1. The following sequences were used: Fluid Acquisition Inversion Recovery (FLAIR), with weighted contrast in T2; Turbo Spin Echo, with weighted contrast in T1 and T2; Susceptibility-Weighted Imaging (SWI) and Short T1 Inversion-Recovery (STIR).
Artificial neural network
The program utilized for the construction of ANN was the Qnet for Windows, in order to find the best and most simple architecture of ANN to classify people with MS e control. We used a MLP type of ANN powered ahead and trained with error back propagation algorithm. Training, cross validation, and testing were performed separately. All data were randomly divided into three subgroups, as follows: 12 people for training; six people to the cross-validation; and six ones for the test. As input parameters of the ANN we used the CS observed values from the tested spatial frequencies (angular frequency stimuli and sine-wave gratings). When the values of y in the ANN are y <0.5, the output of the ANN is considered of value 0 (zero), which would be the rating for the control group. When we get the value y > 0.5, the output of the ANN is considered the value of 1 (one) i. e., the group with MS.
Statistical analysis
Data were analyzed with Statistica software (version 10, Tulsa, Oklahoma, USA), and expressed as mean and standard deviation. For discrete variables, we used the Mann-Whitney U. We also performed multiple regressions considering years of study and the ratings of the neuropsychological tests; between the GCC and ONH with CS; between D15d and CS; and between the CS and the stroop test. CS analysis of the groups with and without MS was performed with the t-Student. In order to ascertain the weight of each factor (frequencies of 1, 3, 4, 24, and 48 cycles/360º and of 0.5, 1.0, 3.0, 4.0 and 7.5 cpd) as input parameters of the ANN, an analysis of covariance (ancova) was performed.
The years of study (p=0.647) of the participants were equivalent and, we assume, did not impact the results of neuropsychological tests. The stroop test was the only neuropsychological test to yield differences between the groups with and without MS. However, there was no correlation between the stroop test and the CS. There was no difference between the CS data and OCT results with the protocols GCC (p=0.766) and ONH (p=0.740); or between CS and D15d (p=0.556). Figure 1 shows the observed curves for the angular frequency stimuli and sine-wave gratings within groups with MS, MSON, and CG. The CS was better in the CG, followed by MS and MSON. The analysis with t-test for independent samples showed that the group MSON was less sensitive, or with smaller CS values for all tested classes of spatial frequencies. The CS for the angular frequency stimuli is higher than that for the sine-wave gratings as seen in the y-axis in logarithmic scale for the two classes of stimuli.
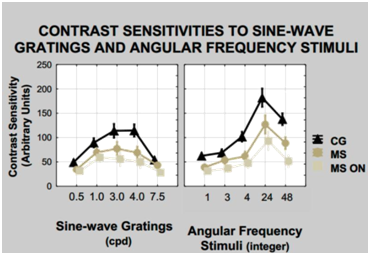
Figure 1 Curve of SC for the stimuli sine-wave grating, to the left and to angular frequency stimuli, right MS, MSNO and control. The error bars standard errors of the mean for each frequency. The t test for independent samples analysis showed differences p<0.001 at all frequencies of the sine-wave gratings (left) and the angular frequency stimuli (right) between the control group and the MS and the MSON.
Results for the angular frequency stimuli showed differences at all frequencies 1, 3, 4, 24, and 48 cycles/360º, for the CG, MS and MSON (p<0.0001). In the interaction MS versus MSON, there were differences in the frequencies of 1, 3, and 48 cycles/360º for the right eye, and at all frequencies of 1, 3, 4, 24, and 48 cycles/360º, for the left eye. The frequency of 24 cycles/360º is the one that best differentiated the groups surveyed. On the other hand, the results of CS with sine-wave gratings show differences at all tested frequencies 0.5, 1.0, 3.0, 4.0 and 7.5 cpd, when MSON and CG were compared, and also when MS and MSON were compared (p <0.0001). Comparing MS and CG yielded differences spatial frequencies of 0.5, 3.0, 4.0 and 7.5 cpd for the right eye (p < 0.0001), and of 0.5, 1.0, 3.0 and 4.0 cpd, for the left eye (p < 0.0001). The spatial frequency of 3.0 cpd was found to be of maximum of sensitivity for the groups with MS and MSON, and declines at 4.0 cpd, as compared to the CG (Figure 1). The spatial frequency of 4.0 cpd was the one that best differentiated the three groups surveyed.
Results from ancova showed correlation at frequencies interactions of 1 - 48, 3 – 4, 24 - 48 cycles/360° (p <0.001). On the other hand, there was correlation in the interactions among spatial frequencies of 0.5 - 3.0 cpd, 0.5 - 4.0 cpd, 0.5 - 7.5 cpd, 3.0 - 4.0 cpd, 3.0 - 7.5 cpd and 4.0 - 7.5 cpd (p <0.001). Thus, the performance of a MLP ANN was evaluated to classify individuals with MS or controle using data obtained from the frequencies of 1, 4 and 24 cycles/360º and from the frequencies of 0.5, 1.0 and 4.0 cpd. This ANN was named “full” to contain the most relevant spatial frequencies, identified by the ancova, as the ANN input parameter. The frequency of 24 cycles/360º and the frequency of 4.0 cpd were included in the construction of a simpler ANN, and their performance was compared to the “full” ANN. Six different types of ANN architectures were built in order to select the one with the lowest mean squared error (ɛ) and better correlation value in the test phase. All those architectures follow a topology with an input layer, two hidden layers, and an output layer. Architecture variations were given by the number of input parameters, the number of neurons in each layer, and the transfer functions of hidden layers and the output layer, either sigmoid, Gaussian, or hyperbolic. The total number of ANN layers is four: the first, an input; the second and third, hidden ones; and the fourth, an output.
Results of ɛ and of ANN correlation 412661-where the number 4 is the amount of ANN layers; number 12 symbolizes input parameters, which, in this case, are CS values obtained with the six frequencies: 1, 4, and 24 cycles/360º, and 0.5, 1.0, and 4.0 cpd, with right and left eyes; digits 66 represent the number of neurons on the second and third hidden layers; and number 1 is the output layer with just one neuron-were 0.0001 and 1, respectively, for the training phase; and 0.25 and 0.71 for cross-validation phase. Result ɛ < 0.10 is considered as excellent; between 0.10 < ɛ < 0.30 is good; between 0.30 < ɛ < 0.50, is regular; and ɛ < 0.50, bad. The maximum correlation value is 1. In an attempt to select an ANN architecture using the CS of only one spatial frequency sine-wave gratings and one spatial frequency of angular frequency stimuli, we built a topology 44221 with the parameters 24 cycles/360º and 4.0 cpg that best differentiate MS from the CG. Results for ɛ and for the correlation in the training phase were 0.001 and 0.99, and cross-validation phase were 0.31 and 0.66 under the same conditions of the ANN architecture 412661. Classification results for the ANN 412661 in the test phase were 0.39 and 0.72 for the ɛ and for the correlation, respectively. The final results of the ANN 44221 to ɛ and for the correlation were 0.40 and 0.71, respectively. Performances of the two ANNs were also estimated as to the degree of sensitivity and specificity within which adults with MS were distinguished from controls (Figure 2&3). The area under the curve ROC for ANN 412661 was 0.9792 and that for ANN 44221 was 0.8958 with a 95% confidence interval. The difference between the two ANNs was not significant p<0.231.
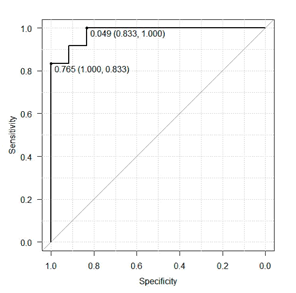
Figure 2 Results from the ROC curve for evaluating the performance in distinguishing patterns of MS as shown by ANN 412661.
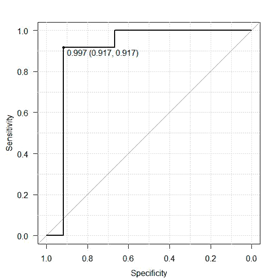
Figure 3 Results from the ROC curve for evaluating the performance in distinguishing patterns of MS as shown by ANN 44221.
This study evaluated the CS of adults with MS, MSON and CG. As for Regan et al.25 and Vieira-Gutemberg et al.26 CS is reduced in MS. The introduction of the RNA technology to classify adults with MS and control using CS parameters, and shortening time, reinforces the importance of employing the CS assessment in the context of clinical MS. Neuropsychological tests also have singular importance in the assessment of people with MS. The literature already recognizes the possible impairment of attention, memory and executive functions caused by MS.33,35 The battery of tests used in this study identified losses in response inhibition, in the stroop test,37 in people with MS. The presence or not of other cognitive impairments depend on variables such as years of study, profession and occupational activity, time and severity of the disease and the existence of other co-morbidities. These results are equivalent to findings of Bonnet et al.42 who also found no difference between people with MS and CG when years of study were high. For these authors, there seems to be a cognitive compensation in people with MS and higher levels of education. Our research adopted very strict criterion to select a CG with the same educational level as well as professional to be equivalent to the MS group with the main purpose of understanding, on the onset of the deficit of attention, memory and/or executive functions investigated, and if such deficits affect the results of CS to spatial frequencies. The results found with GCC and ONH protocols of OCT, with D15d and with the stroop test allowed understanding that, for the examined population, the losses in CS found in MS appear not to be affected by these factors and tests; although results of the stroop test yielded differences from the CG.
Even though some volunteers had history of ON, evaluation of GCC and ONH did not identify injury, perhaps due to the time and the severity of the disease. The EDSS of participants with MS had a maximum 6.0 and an average of 2.5±1.41 standard deviation and a maximum of 14 years of illness and average 6.89 years ±4.11 standard deviation. Similarly, to the findings of Serbecic et al.43 the time the illness seems not to have correlation with changes in OCT. The absence of correlation between the CS data, measured with a psychophysical method, and the anatomy of retinal nerve fibers measured by OCT, can be explained by the coexistence of damage to the retina and retinal projections.19 Also, other visual tests can quantify visual dysfunctions in the absence of damage to the retinal structure by reflecting the involvement of visual pathways after the optic nerve.18
The losses in CS found with psychophysical paradigms, and consecutive visual pathways lesions often do not come with a reduction in retinal nerve fibers layer as measured by OCT. Small measurement differences, and physiological variations in the thickness of the layer of nerve fibers when eyes and subjects are compared, or, as in the present research, the small size of the sample could contribute to the reduced correlation. The prevalence of MS in the city of João Pessoa, where volunteers were selected, totals 90 people notified until March 2014. Of this total, only 68 are in the range of 20 to 50 years and of this total, 36 did not meet the research criteria. From the remaining 36 volunteers with MS and MSON, and only 12, participated in all stages of the research.44–48 To build both the ANN 412661 and of the ANN 44221 this same sample had to be used, the same number of layers and the same sigmoid transfer function. The number of connections and interactions of artificial neurons differed depending on the total number of input parameters of the ANN, the total number of neurons, and on the best stopping point of the curve of ɛ, and on the cross-validation phase of each architecture as well.
The results of both, ANNs 412661 and 44221, presented good capability of classification of patterns with correlations equal to 0.72 and 0.71, respectively, in test phase. Future research shall point whether the use of ANN is useful in classifying the benign forms of MS, and in monitoring the progression of MS through the CS. The simple architecture of ANN 44221 succeeded in classifying people with and without MS using spatial frequencies of patterns, 24 cycles/360º and 4.0 cpg showing results equivalent to the ANN 412661. There was no difference between the areas under the curves ROC for the two ANNs, 44221 and 412661. This fact allows us to infer that the addition of CS to the angular frequency stimulus 24 cycles/360º is quite relevant to the early assessment of adults with MS. This research continues to pursuit the objective of reducing the time to measure CS with the psychophysical method of forced choice and suggest the use of this assessment in the clinical practice and the study of MS.
We thank the following funding agencies: the National Research Council (CNPq), Coordination for the Improvement of Higher Education’s Human Resources (CAPES) and Reference Center for Multiple Sclerosis Paraíba (RCMSPB).
The author declares there is no conflict of interest.

©2015 Guimaraes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.