Advances in
eISSN: 2377-4290


Research Article Volume 8 Issue 1
1Ophthalmology Department, College of Medicine, King Saud University, Saudi Arabia
2Pathology Department, King Saud University-Medical City, Saudi Arabia
3Pathology and Laboratory Medicine Department, King Khaled Eye Specialist Hospital, Saudi Arabia
4Wilmer Eye Institute, Johns Hopkins University School of Medicine, USA
5Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago, USA
6Moorfield’s Eye Hospital Centre, UAE
Correspondence: Hind Manaa Alkatan, Department of Ophthalmology, College of Medicine, King Saud University, Riyadh, Saudi Arabia, Consultant: Department of Pathology, Director of KSU Residency, Training Program in Ophthalmology, College of Medicine, King Saud University, Riyadh, PO Box 18097, Riyadh, postal code 11415, Saudi Arabia, Tel +966504492399, Fax +966112052740
Received: February 11, 2018 | Published: February 21, 2018
Citation: Alkatan HM, Alotaibi MD, Maktabi AY, et al. Immunohistochemical characterization of sub retinalmembranes (SRMs) in proliferative vitreoretinopathy. Adv Ophthalmol Vis Syst. 2018;8(1):60-63 DOI: 10.15406/aovs.2018.08.00270
Purpose: To provide immuno histo chemical characterization of sub retinal bands removed during retinal surgery in eyes with proliferative vitreo retinopathy (PVR).
Methods: This study included all eyes with the clinical diagnosis of PVR that underwent pars plana vitrectomy surgery during which the subretinal tissue causing retinal detachment was obtained. The subretinal bands were removed “en bloc” through retinotomy using subretinal intraocular forceps. The excised tissue was sent for histopathologic analysis. Immunohistochemistry (IHC) was performed to confirm the cellular nature and components of these subretinal membranes. The IHC stains included, glial fibrillary acidic protein (GFAP), Pancytokeratin, CD3 CD20 CD68 and CD34.
Results: Subretinal membranes (SRMs) from 7 eyes were included in the analysis. All cases had successful surgical outcome with reattachment six months after surgery. The microscopic examination of the excised tissue nicely demonstrated the constituents of the SRM as follows: retinal pigmented epithelial (RPE) cells that stained positively with cytokeratin (7/7), avascular plaques of RPE cells showing metaplasia in the form of spindle cells (7/7). Fragments of gliotic GFAP-positive neural retina was adherent to the fibrous plaque (6/7). Bruch’s membrane was identified in one specimen. CD68 positive macrophages were seen in (5/7) being silicon oil- laden macrophages in2/5. Rare CD3 positive cells were also noted in 1 specimen.
Conclusion: Subretinal bands in PVR are mainly composed of reactive avascular plaques of RPE metaplasia and macrophage infiltration. The overlying gliotic retina or Bruch’s membrane are likely to be adherent to such plaques and might be inadvertently excised during removal of such membranes. Removal of SRMs is essential for successful reattachment of the retina.
Proliferative Vitreo retinopathy (PVR) describes the process of ectopic cell proliferation that follows rhegmatogenous retinal detachment (RRD). This proliferation commonly occurs in the vitreous or the peri retinal area.1 The retina society first described the term Proliferative Vitreo retinopathy (PVR) in 1983.2 The disease was later classified by the Silicone Study Group into different groups based on the severity (minimal to massive), location of the proliferation (anterior to posterior), and the type of the contraction (diffuse, focal, sub retinal, and circumferential).3A grading system was also introduced to PVR that includes grade A, B, and C. In this study we are mostly interested in grade C that represents PVR with sub retinal membranes (SRM) or bands. 4 We describe the histopathological and immunohistochemical features of such a proliferation with summary of the main difference in the constituents between SRM and Epi retinal membranes (ERM).
This study included eyes with the clinical diagnosis of PVR that underwent pars plana vitrectomy (PPV) surgery at King Khalid Eye Hospital. During PPV the sub retinal bands associated with retinal detachment were obtained. The subretinal bands were removed “en bloc” through a retinotomy using sub retinal intraocular forceps. The excised tissue was fixed in formalin and sent for routine histopathological analysis. 2 Pathologists reviewed the histopathological routine slides stained with Hematoxylin and Eosin to confirm the diagnosis of sub retinal band. Immunohistochemistry (IHC) was performed to confirm the cellular nature and components of these sub retinal membranes. Positive staining to each of the constituents was checked and confirmed. Data were entered though excel where any further editing were carried out before interpreting the results.
We included 7 cases with the tissue diagnosis of a sub retinal membrane. The histopathological appearance was variable; however, the major constituents were RPE cells and proliferating fibrous tissue being constantly observed in all 7 cases (Figures 1A&B). Avascular plaques of RPE cells showing metaplasia in the form of spindle cells that resembled fibroblasts with relatively low proliferation index was seen (Figure 1C). The RPE cells stained positively with cytokeratin in all cases (Figure 1D). Fragments of gliosis with documented GFAP positive neural retinal tissue was adherent to the fibrous plaque in 6 cases (Figure 1E). CD68 positive macrophages were seen in 5/7 specimens and 2 out of these specimens (2/5) showed silicon oil- laden macrophages (Figure 1F & 1G). Rare CD3 positive cells representing reactive T-lymphocytes were also noted in 1 specimen (Figure 1H). Bruch’s membrane was identified in one specimen (Figure 1I). All cases had successful surgical outcome with reattachment six months after surgery.
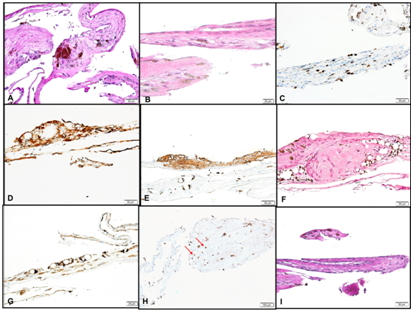
Figure 1 (A) Sub retinal membrane in one of the cases consisting mainly of RPE cells, and fibrous membrane. (Original magnification X200 Periodic Acid Schiff). (B) Another area in the same membrane with fibroblastic metaplasia (Original magnification X400 Hematoxylin & Eosin). (C) The same area of fibrous tissue formation showing low to moderate proliferation index (Original magnification X200 Ki67). (D) RPE cells in another case of sub retinal band following RRD staining positively with the Pan cytokeratin stain (Original magnification X200 CytoK). (E) The gliotic portion of the membrane in the same case showing positive IHC staining (Original magnification X200 Glial Fibrillary Acidic Protein). (F) A sub retinal membrane with RPE cells, and macrophages surrounding silicon oil empty spaces. (Original magnification X200 Hematoxylin and Eosin). (G) The macrophages surrounding the silicon oil droplets show positive staining with IHC marker in the same specimen above (Original magnification X200 CD68). (H) Few scattered reactive T lymphocytes showing positive IHC staining (Red arrows). (Original magnification X100 CD3). (I) The sub retinal band with enclosed portion of Bruch’s membrane. (Original magnification X200 Periodic Acid Schiff).
After the development of RRD a breach in the blood-retinal barrier (BRB) and the inflammation cascade occur. Chemotactic agents, growth factors and cytokines leaking through the affected blood retinal barrier contribute to the proliferation of ectopic cells in the periretinal area. Our study is mainly concerned about the subretinal bands or SRM that form after such proliferation. Studies that have been conducted recognized that the main constituent of these bands are retinal pigmented epithelium (RPE), glial cells, fibroblasts, and macrophages among others, which usually migrate through the affected BRB that happens after RRD.5−7 The mainstay of treatment in PVR is surgery. Surgical treatment is focused on relieving the traction forces and closing the break trying to restore the anatomical features of the affected eye.8 This depends on the severity and grade of the disease. The use of vitrectomy with tamponade, scleral buckling and retinotomy depends also on the severity of the case. Generally speaking these procedures tend to have unsatisfactory success rates if used alone.9 Knowing the immunohistochemical characterization of sub retinal bands will provide us with a better understanding on how to prevent PVR by allowing early intervention to take place.10 The IHC stains, which are useful include: glial fibrillary acidic protein (GFAP), pan cyto keratin, CD3 CD20 CD68 (to highlight inflammatory cells: T-lymphocytes, B-lymphocytes and macrophages, respectively) and CD34.11
Our findings concur to what has been described by Matsumura, where it has been recognized that RPEis the most prominent cellular component in their studied cases.5 Hiscottsimilarly found that glial cells had a non-negligible role in the development of SRM but may not be found in all cases. In our series, glial component was observed in all cases except one. It is also commonly accepted that such bands in PVR are not vascularized.6 Other studies suggested that the traction forces of the bands which prevents reattachment is mainly caused by the presence of fibroblast, various types of collagen and extracellular matrix component of the bands illustrating the necessity of removing such bands to aid in the process of successful reattachment. The phenotypes of the migrated RPE cells in affected eyes include fibrous metaplasia and macrophage-like cells.
Bruch’s membrane can be a component in SRMs in contrast to the presence of the PAS-positive internal limiting membrane (ILM) in ERM.12 Bruch’s membrane has been observed in one case only (1/7) in this series with absence of ILM as expected. A simple idiopathic ERM is usually non-contractile and is composed of Muller cells while complex ERMs are contractile and contain fibroblasts, which might produce traction forces on the retina depending on the underlying ophthalmic condition.13 Secondary ERMs show access of RPE with different proliferation index and characteristic Nestin-positive cells.14,15 In contrast, RPE proliferation and metaplasia have been the main histopathological features in SRM with major contribution to its pathogenesis.16−19 Macrophages and other inflammatory cells such as lymphocytes are major components in various types of SRMs and are also seen in ERMs more commonly in eyes with uveitic entities.17,18,20,21This has been observed in our series with predominant macrophages in 5 cases, 2 of which were related to the use of silicon oil in addition to reactive T-lymphocytes detected by CD3 IHC stain in 1/7. Table 1 summarizes the possible differences in the cellular components between epi-retinal and sub-retinal membranes; however the description of ERM formation and the detailed differences between idiopathic and secondary ERMs is beyond the scope of this paper.
|
Component |
Epi-retinal membrane (ERM) |
Sub-retinal membrane (SRM) |
|
RPE proliferating cells |
Mostly absent in Idiopathic ERM Present in Secondary ERM (post-RD/PDR) by migration of RPE. Detected by Cyto keratin and Ki67 stains. |
Predominant May be trans differentiated with a giogenic role. |
|
Glial tissue |
Present as major component in both Idiopathic and secondary. Detected by GFAP stain. |
Absent May be present as a less prominent component |
|
Fibroblasts |
Present as non-epithelial fibroblasts in both Idiopathic and secondary. Detected by SMA stain. |
Present as RPE-derived contractile myo fibroblasts especially in PDR and chronic RD. |
|
Endothelial cells |
Absent in idiopathic ERM Present in secondary ERM related to PDR. |
May be a vascular.
|
|
Internal limiting membrane |
Present. Detected by PAS stain. |
Absent |
|
Inflammatory cells |
Present in association with uteritis and in secondary ERM in relation to Cytokines release.
|
Abundant, predominantly macrophages and also include T-lymphocytes. Detected by CD68 and Lymphocytic markers: CD3 and CD20. |
Table 1 The cellular components of epi-retinal membranes compared to sub-retinal membranes
RD, retinal detachment; PDR, proliferative diabetic retinopathy; GFAP, glial fibrillary acidic protein;
SMA, smooth muscle actins; PAS, periodic acid schiff
Matrix metalloproteinases (MMPs) are other important components of sub-retinal and epi-retinal bands, which are worth mentioning but were not the focus of this current study. These enzymes contribute to the contractile features of these bands. Webster looked at the various MMP expressions in both sub-retinal and Epi-retinal bands and found that MMP-2 is more prominent in Epi-retinal bands compared to SRMs, which might signify that these enzymatic interactions happen more frequently at the vitreoretinal interface compared to the sub-retinal area.22 Surgical removal of such bands is agreed upon but the development of new pharmacological drugs, a great interest is now focused on the pharmacologic management of PVR. Trying to prevent inflammation with subsequent cell migration and proliferation is the idea behind most medication.19,23 Low dose intra vitreal triamcinolone, anti-proliferative and anti-neoplastic medications have shown some benefit in PVR if combined with surgery.24
None.
The authors declare that there is no conflict of interest.
None.

©2018 Alkatan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.