Advances in
eISSN: 2377-4290


Review Article Volume 13 Issue 3
1Department of Ophthalmolgy, Minia University, Egypt
2Department of Ophthalmolgy, Zembely Eye Center, Egypt
Correspondence: Hosam A Ibrahim Elzembely, Ophthalmolgy Department, Zembely Eye Center, Minia University, Egypt
Received: September 07, 2023 | Published: October 5, 2023
Citation: Amer MR, Elzembely AI. Evolution of penetrating keratoplasty: the three eras, a brief historical review. Adv Ophthalmol Vis Syst. 2023;13(3):86-88. DOI: 10.15406/aovs.2023.13.00447
Corneal blindness is responsible for approximately 10% of blindness cases worldwide. More importantly, in most developing countries, corneal diseases represent the second leading cause of blindness. The history of corneal transplant surgery is an intriguing and fascinating story of more than 200 years duration filled with wild theories, animal experiments (xenograft), human trials, colorful adventurism, and serious clinical science. Early fascination with the idea of restoring clarity to an opacified cornea first emerged in the writings of Galen (130-200 AD), who suggested the concept of restoring the transparency of an opaque cornea. He recommended a form of superficial keratectomy (abrasio corneae). The initial corneal transplant was conducted in 1824, almost 190 years ago, by Reisinger. Thus, development of corneal transplant surgery can be divided into 3 eras: xenograft era, surgical era and femtosecond era.
The structure known as the ocular wall is composed of three distinct layers, namely the fibrous layer, vascular layer, and retina, also referred to as the nerve layer. The fibrous layer is comprised of the cornea, which facilitates vision by refracting light, and the sclera, also known as the "white" of the eye. The sclera, a fibrous tissue that envelops the entirety of the eyeball with the exception of the cornea, serves as a protective barrier for the eye. The intermediate stratum of the ocular structure, referred to as the vascular tunic, encompasses the vascular choroid coat, the ciliary body and the iris, also known as the pigmented region of the eye. The pupil is located in the central position within the iris. The initiation of the visual pathway occurs within the retinal layer of the eyeball; this particular layer predominantly comprises epithelial cells that possess melanin. The presence of melanin in the retina serves to mitigate the adverse effects of glare, so preserving the integrity of visual perception. The formation of retinal images occurs as a result of the refraction of light rays by the cornea and lens.1
The cornea as part of the outer tunica has a curved morphology and transparent composition thus facilitating the process of light transmission and focusing. The cornea is avascular; this means that it is immuno-privileged rendering the antibodies responsible for transplant rejection incapable of accessing the corneal tissue. The rate of rejection in human corneal transplants is generally below 5%, making corneal transplants the most efficacious form of transplant surgery.2 On average, the horizontal corneal diameter in adults ranges from 11.5 to 12.0 mm, which is approximately 1.0 mm larger than the vertical diameter. The corneal optical system demonstrates asphericity due to its prolate form, steep center, and flat periphery. The variation in the organization of the anterior stroma contributes to its high cohesive strength and potentially elucidates the greater resistance of the anterior curvature to changes in stromal hydration compared to the posterior stroma. The human cornea is composed of five distinct layers (Figure 1). Among these layers, three are cellular in nature, while the remaining two layers consist of interfaces. Additionally, there is a newly discovered layer known as Dua's layer.3 The epithelium has a thickness of approximately 50 μm, while the stroma ranges from 450 to 500 μm in thickness. Bowman's layer is relatively thin, measuring around 8 to 10 μm, while the endothelium is approximately 5 μm thick. Furthermore, there exists a layer known as Dua's layer and Descemet's membrane, which are situated between the stroma and the endothelium. The central region of the cornea often has a lesser thickness compared to its peripheral regions. The epithelium consists of a stratified squamous epithelial cell layer, often comprising 5-7 layers, which are nonkeratinized. Bowman's membrane comprises Type I and V collagen, along with proteoglycans. It is situated anterior to the stroma. Descemet's membrane (DM) is composed of Type IV collagen and laminin. The endothelium consists of a monolayer of hexagonal cells.4
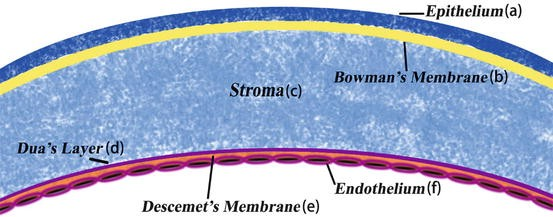
Figure 1 Layers of the cornea.5
The three eras of development of penetrating keratoplasty
The scientific activity and achievement of corneal graft throughout history could be plotted into three distinctive ages or eras. The way of thinking and the technological advances were at the heart and peculiarities of each era.
The first era; xenograft era
Erasmus Darwin (1731-1802), the grandfather of Charles Darwin, takes the credit for first mentioning the concept of corneal trephination as part of the procedure to manage leucomatous cornea in 1760. The 19th century was filled with experimentation and frustration. In 1824, Franz Reisinger coined the term keratoplasty and proposed the concept of replacing a scarred cornea with a transparent animal cornea (corneal xenograft). It is possible that James Bigger was the pioneer in performing a keratoplasty procedure, when he replaced the opaque cornea of a pet gazelle with a translucent cornea obtained from another gazelle.
The second era; surgical keratoplasty
After the initial 30 years of the 19th century, interest in keratoplasty declined, and pioneers then concluded that it was simply an audacious fantasy. In 1905, from the small town of Morava in the Czech Republic came the first report of a successful penetrating keratoplasty by Edward Konrad Zirm.4 Although this surgical procedure did not signal the onset of unqualified success in the field of corneal transplant surgery, this single case report certainly rekindled the interest among his colleagues to further refine this procedure. A few years later, in 1914, Anton Elschnig, who is first to suggest use of cadaveric tissue, developed partial penetrating keratoplasty as a viable surgical option using the von Hippel trephine (Figure 2), a device developed by Von Hippel in 1888 for the purpose of corneal trephination, specifically targeting 5 mm corneal zone. In 1930, Elschnig presents 20 years data of 170 corneal transplants with 22% overall success rate. Townley Paton and John McLean established First Eye Bank in New York in 1944.6

Figure 2 Von hipple first corneal trephine.7
In 1963, Richard Troutman introduced the microscope and sutures for keratoplasty to the United States. The progressive development and refinement of surgical instruments and suture materials have contributed to the gradual improvement and advancement of keratoplasty procedures.8 The commercial introduction of 10-0 nylon by Johnson & Johnson in 1968 led to its adoption by ophthalmologists for use in keratoplasty surgery, representing a noteworthy progression in the field. The demand for corneas derived from diseased individuals experienced a surge in response to the advancements and growing efficacy of corneal transplantation procedures. In the United States, over 36,000 corneal transplant procedures were conducted in 2005, employing corneas sourced from the Eye Bank Association of America. Furthermore, an extra 9,000 corneas were exported for transplantation purposes overseas. The concept of corneal storage in artificial media was initially introduced by McCarey and Kaufman during the early 1970s.9
The third era; femtosecond era
Femtosecond laser technology was first developed by Dr. Kurtz at the University of Michigan in the early 1990s.10 It was rapidly adopted in the surgical field of ophthalmology. Femtosecond lasers emit light pulses of short duration (10−15 s) at 1053 nm wavelength that cause photo-disruption of the tissue with minimum collateral damage. This enables bladeless incisions to be performed within the tissue at various patterns and depth with high precision. This review paper presents the most recent advancements in femtosecond lasers in modern corneal and refractive surgery.11
Penetrating Keratoplasty (PKP) has changed over the years, from the early 1900s, when the first procedure was performed without any modern surgical device12 to the novel femtosecond laser-assisted PKP concept which is documented for use in keratoplasty in the year 2000. On the other hand, optimal postoperative outcomes remain a concern as they are dependent on a centered and perpendicular cut of the recipient cornea, and a well-matched donor button and recipient bed.13 The femtosecond laser has shown promise in improving postoperative outcomes, as it can achieve a greater precision in cutting either the donor or the recipient cornea, minimize misalignments and increase the stability of the wound.14 The concept of a stepped graft edge dates back to 1960s, when Jose I. Barraquer described the “two-level keratoplasty” technique, characterized by a difference in the size of the graft at the level of the anterior and posterior layers of the cornea.15 Over the years, technical complexity and manual techniques have prevented the shaped corneal grafts from becoming widely implemented.16 However, the femtosecond laser has now enabled the creation of advanced shaped corneal cuts, eliminating manual dissection.13
Laboratory studies in shaped corneal grafts using femtosecond lasers first demonstrated the mechanical stability of different known wound configurations, such as “top-hat”, “mushroom”, “zigzag” and "Christmas tree".14 Subsequent in vivo reports were very optimistic in describing excellent wound apposition, wound integrity and best spectacle-corrected visual acuity greater than 20/30, after 6 months, in patients who underwent PKP with femtosecond laser zigzag pattern.17 Likewise, the first study comparing the conventional blade trephination versus the femtosecond laser generated zigzag incision, demonstrated consistently lower induced astigmatism in the laser group. The greatest difference was experienced at month 1, followed by month 3, when the average astigmatism was 4.5 D in the conventional group versus 3.0 D in the laser group (p = 0.018).16 The femtosecond laser allows for patterns and angles of incisions that are not achievable with conventional trephines.16 A prospective study following patients for one year, showed the laser efficacy in creating precise and complex wound configurations, even when significant corneal opacity was present. In addition, the typical increased wound healing area in the top-hat configuration enabled faster and secure suture removal.18 Regular and irregular astigmatism remain a major postoperative challenge in full thickness keratoplasty. A recent study has shown favorable outcomes of “mushroom” femtosecond laser-enabled keratoplasty (M-FLEK), in contrast to conventional PKP in eyes with keratoconus. Decreased astigmatism was observed despite no significant differences in best-corrected visual acuity.19 The field of ophthalmology is currently entering a new phase characterized by the adoption of femtosecond laser-assisted keratoplasty. The utilization of the femtosecond laser in corneal surgery is seeing significant and extensive recognition. Previous studies have reported favorable outcomes in terms of early visual rehabilitation and reduced occurrence of both short-term and long-term complications in young patients who underwent femtosecond laser assisted keratoplasty.20 The progress of science is a continuum. The three eras fore-mentioned are only part of that continuum (Figure 3). The future is always exciting to see and predict. Scientists hold the torch and lead humanity. The third era is only the beginning of another era paved by further developments in basic sciences.
None.
The authors declares that there are no conflicts of interest.
None.

©2023 Amer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.