Advances in
eISSN: 2377-4290


Research Article Volume 6 Issue 5
1Department of Ophthalmology Indus Medical College Hospital Pakistan
2Students at Isra University Hospital Pakistan
Correspondence: Nisar Ahmed Khan Assistant Professor Consultant Ophthalmologist Indus Medical College Hospital Tando Mohammad Khan Pakistan, Tel +92-300-3014168
Received: March 30, 2017 | Published: April 12, 2017
Citation: khan NA, Khan AA, Khan A, Khan A, Memon JI (2017) Ranibizumab in the Treatment of Chronic Central Serous Chorio Retinopathy. Adv Ophthalmol Vis Syst 6(5): 00196. DOI: 10.15406/aovs.2017.06.00196
Purpose: To evaluate the efficacy of ranibizumab in the treatment of chronic central serous chorio-retinopathy at our department of ophthalmology, Indus Medical College Hospital Tando Mohammad Khan. Pakistan.
Introduction: Central serous chorio-retinopathy is self-limited in the majority of patients, who usually retain excellent vision. However, those who do not resolve spontaneously may develop permanent visual impairment because of pigment epithelial and photoreceptor damage. Most clinicians prefer to observe these patients for three months before considering any treatment options because most cases recover spontaneously. Infrequently, neurosensory retinal detachment persists and leads to RPE and photoreceptor damage. This form of the disorder is called chronic central serous chorio-retinopathy (CCSC) and can result in severe effects on macular function. The pathogenesis of chronic central serous chorio-retinopathy (CCSC) remains indistinct. Vascular endothelial growth factor (VEGF) is produced by retinal and choroidal cells, increases vascular permeability and leads to edema by uncoupling the endothelial cell to cell junctions. For this reason, anti-VEGF agents are used therapeutically to reduce or reverse the choroidal leakage in acute CSC. Intravitreal bevacizumab a humanized monoclonal VEGF antibody was reported to be associated with favorable outcomes without adverse events in patients with acute CSC. Ranibizumab is another anti-VEGF agent which has potentially better retinal penetration than bevacizumab because of its smaller molecular size and higher binding affinity for VEGF. Ranibizumab is a recombinant humanized antibody fragment that blocks vascular endothelial growth factor (VEGF). It is injected intravitreally as a treatment for exudative age-related macular degeneration, diabetic macular edema, and macular edema secondary to central and branch retinal vein occlusion.
Material and methods: It is a retrospective, non comparative interventional study conducted from September 2015 to December 2016. This study includes 7 eyes of 7 patients, who were diagnosed as suffering with chronic central serous chorio-retinopathy for more than 6 months and were not using any treatment during last 3 months. Patients were diagnosed relying upon history, visual acuity using standard Snellen chart, dilated fundus exam using slit lamp with 90D lens and OCT (ocular coherence tomography) findings. All were male patients with mean age of 35 years, 5 were suffered with left eyes while remaining 2 were suffered with right eyes. All were having visual issues (decrease/distortion), vision was ranging between 0.5 to 0.3 average vision was 0.37. Macular thickness was variable ranging between 253 to 376 average 290.28microns. All 7 eyes were treated with intravitreal injection of ranibizumab 0.5 mg/0.05 ml (Lucentis®; Novartis), only one injection used after aseptic measures, draping, and povidone-iodine 5% solution used for periocular area as well as conjunctival sac and copious irrigation done after 3 minutes in operating room under Takagi microscope. Eye was patched for 2 hours and patient discharged only on moxifloxacin eye drop 6hourly for 7 days. Patients followed after 3rdday, 15th day and 30th Visual acuity recorded, dilated fundus exam carried out and OCT done on each visit respectively.
Results: All 7 eyes were received Intravitreal injection of ranibizumab 0.5 mg/0.05 ml, only one injection used and patients followed on 3rd, 15thand 30th day respectively. Visual acuity recorded, dilated fundus exam carried out and OCT done on each visit. Out of 7 eyes 5 improved vision to 0.8 and macular thickness decreased to 223 (±5) microns on 3rd On 15th day all eye improved vision and becomes 1.0 along with macular thickness 214 (±2) microns that further reduced to 210 (±1) microns on 30th day.
Conclusion: Though it is still not clear that VEGF has some role in inducing central serous chorio-retinopathy but the use of anti VEGF for its treatment giving results. In our study, we used only one injection of ranibizumab 0.5mg/0.05ml with excellent results in improving vision and reducing macular thickness with in the period of 15 days with no recurrence until now.
Keywords: khan na, khan aa, khan atiqa, khan aisha, memon ji, treatment, chronic central serous chorio-retinopathy (CCSC), ranibizumab, indus medical college hospital
CCSC, chronic central serous chorio-retinopathy; VEGF, vascular endothelial growth factor; CSC, central serous chorio-retinopathy; RPE, retinal pigment epithelium; PDT, photodynamic therapy
Central serous chorio-retinopathy (CSC) is characterized by serous retinal detachment with dysfunction of the retinal pigment epithelium (RPE) and choroid.1 It typically occurs in patients between the ages of 30 and 50 years and affects men more often than women.2 A variety of risk factors have been associated with CSC, including psychological stress, type A personality, hypertension, systemic steroid usage, pregnancy, and genetic susceptibility.3 The exact pathogenesis of the disease is not fully understood, but abnormalities in the inner choroidal layers, such as venous congestion, ischemia and/or inflammation, lead to choroidal hyper permeability, secondary RPE damage, and serous detachment of the neural retina.3‒5 CSC is self-limited in the majority of patients, who usually retain excellent vision. However, those who do not resolve spontaneously may develop permanent visual impairment because of pigment epithelial and photoreceptor damage.6 Most clinicians prefer to observe these patients for three months before considering any treatment options because most cases recover spontaneously.7‒9 Infrequently, neurosensory retinal detachment persists and leads to RPE and photoreceptor damage. This form of the disorder is called chronic central serous chorio-retinopathy (CCSC) and can result in severe effects on macular function.10 The pathogenesis of chronic central serous chorio-retinopathy (CCSC) remains indistinct.11 Conventional treatment modalities, such as laser photocoagulation or photodynamic therapy (PDT), have been used to treat CSC and it has been demonstrated that these treatment options may be associated with some potential complications, such as RPE changes, choroidal hypo perfusion, and choroidal neovascularization, despite their beneficial effects.12‒14
Vascular endothelial growth factor (VEGF) is produced by retinal and choroidal cells, increases vascular permeability and leads to edema by uncoupling the endothelial cell to cell junctions,15 for this reason, anti-VEGF agents are used therapeutically to reduce or reverse the choroidal leakage in acute CSC. Intravitreal bevacizumab a humanized monoclonal VEGF antibody was reported to be associated with favorable outcomes without adverse events in patients with acute CSC.16‒18 Ranibizumab is another anti-VEGF agent which has potentially better retinal penetration than bevacizumab because of its smaller molecular size and higher binding affinity for VEGF. Ranibizumab is a recombinant humanized antibody fragment that blocks vascular endothelial growth factor (VEGF). It is injected intravitreally as a treatment for exudative age-related macular degeneration,19 diabetic macular edema,20 and macular edema secondary to central and branch retinal vein occlusion.21
It is a retrospective, non comparative interventional study conducted at Indus Medical College Hospital from September 2015 to December 2016. This study includes 7 eyes of 7 patients, who were diagnosed as suffering with chronic central serous chorio-retinopathy for more than 6 months and were not using any treatment during last 3 months. Patients were diagnosed relying upon history, visual acuity using standard Snellen chart, dilated fundus exam using slit lamp ( SHIN-NIPPON SL-203, Japan) with 90D lens and OCT (ocular coherence tomography by NIDEK, model RS-330 Japan) findings. All were male patients with mean age of 35 years, 5 were suffered with left eyes while remaining 2 were suffered with right eyes. All were having visual issues (decrease/distortion), vision was ranging from 0.5 to 0.3 average vision was 0.37. Macular thickness was variable ranging between 253 to 376 average 290.28 microns (Table 1). All 7 eyes were treated with Intravitreal injection of ranibizumab 0.5 mg/0.05ml (Lucentis®; by Novartis Pharma Stein AG, Stein Switzerland), only one injection used after aseptic measures, draping, and povidone- iodine 5% solution used for periocular area as well as conjunctival sac and copious irrigation done after 3 minutes in operating room under Takagi microscope. Eye was patched for 2 hours and patient discharged only on moxifloxacin 0.5% eye drop 6 hourly for 7 days. Patients followed after 3rd day, 15th day and 30th day. Visual acuity recorded, dilated fundus exam carried out and OCT done on each visit respectively (Figure 1).
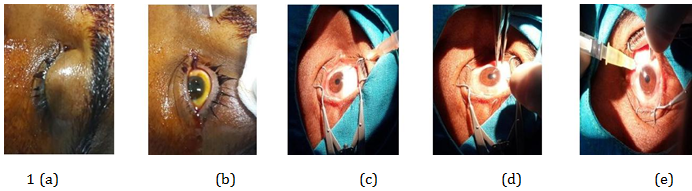
Figure 1 A, B, C, D, and e shows steps during one of our patient receiving Intravitreal injection of ranibizumab 0.5 mg/0.05 ml.
S No |
Age |
Laterality |
Visual acuity |
Macular thickness |
|
Right |
Left |
||||
1 |
27yrs |
R/E |
|
0.5 |
376 |
2 |
28yrs |
|
L/E |
0.4 |
283 |
3 |
31yrs |
|
L/E |
0.4 |
297 |
4 |
35yrs |
|
L/E |
0.4 |
292 |
5 |
39yrs |
R/E |
|
0.3 |
253 |
6 |
42yrs |
|
L/E |
0.3 |
267 |
7 |
43yrs |
|
L/E |
0.3 |
264 |
Table 1 Showing pretreatment evaluation
All 7 eyes were under gone Intravitreal injection of Ranibizumab 0.5 mg/0.05 ml, only one injection used and patients followed on 3rd, 15th and 30th day respectively. Visual acuity recorded, dilated fundus exam carried out and OCT done on each visit. Out of 7 eyes 5 improved vision to 0.8 and macular thickness decreased to 223 (±5) microns on 3rd day. On 15th day all eye improved vision and becomes 1.0 along with macular thickness 214 (±2) microns and reduced to 210(±1) microns on 30th day (Table 2) (Figure 2).
Days |
No of patients |
Visual acuity |
Macular thickness in microns |
3rd |
5(71.42%) |
0.8 |
223(±5) |
15th |
7(100%) |
1.0 |
214(±2) |
30th |
7(100%) |
1.0 |
210(±1) |
Table 2 Showing post treatment evaluation
Chronic central serous chorio-retinopathy (CCSC) is characterized by neurosensory retinal detachment overlying areas of RPE atrophy and pigment mottling, Schaal et al.22 treated 12 eyes with CCSC in which patients received 2±1 Intravitreal injections of bevacizumab (2.5mg) on average during a follow-up of 24±14 weeks. Mean BCVA increased by 2±2 lines. Mean CRT improved significantly over follow-up (P < 0.05), with six patients (50%) showing complete resolution of subretinal fluid. Stewart23 mentioned that laser damages the RPE with a risk of developing choroidal neovascularization, which has left clinicians looking for new treatments that are both more effective and less toxic. Researchers have also used the 810 nm laser, as in transpupillary thermotherapy. However, Penha et al.24 described severe retinal thermal injury in a 31-year-old man following this treatment and recommended caution for these adverse complications. Cardillo Piccolino et al.25 stated that PDT guided by indocyanine green angiography seems to treat macular detachment and serous exudation and preserve visual acuity in patients with chronic central chorio-retinopathy (CCSR). PDT may possibly decrease choriocapillaris blood flow and choroidal exudation in the areas of subretinal fluid production.
However, there are undesired effects such as pigmentary changes in the treatment zone and persistent hypoperfusion of the choriocapillaris. Chan et al.26 stated that development of choroidal neovascularization within the photodynamic region was due to localized ischemia in the choriocapillaris resulting from PDT in chronic central serous chorio-retinopathy (CCSC). Shams & Ianchulev27 stated that VEGF is a regulator of ocular angiogenesis and vascular permeability. When increased choroidal hyper permeability claimed to be one of the main causes of central serous chorio-retinopathy (CSC), it seemed logic to treat such cases with anti-VEGF such as ranibizumab. Kim et al.28 studied the effectiveness of Intravitreal ranibizumab injection for acute central serous chorio-retinopathy (CSC), symptomatic for less than 3 months on 20 patients (0.5 mg/0.05ml) who were followed up for 6 months. All patients had increased BCVA, decreased CRT, and resolution of the neurosensory detachment. In our study, we used only single injection of Intravitreal ranibizumab to treatment chronic central serous chorio-retinopathy (CCSC) and got good results, although there is no such evidence as regards the role of VEGF in inducing central serous chorio-retinopathy (CSC).
Torres-Soriano et al.29 reported in a study of six eyes five chronic and one recurrent central serous chorio-retinopathy (CSC) that visual acuity improved in all cases by 1 month after bevacizumab injection and remained stable up to the third month;. Two eyes (1/3 of patients) required a second injection of bevacizumab with a higher concentration dose of 2.5mg but our results are far superior in only one injection of ranibizumab and there was no recurrence until now. Schaal et al.22 treated 12 eyes with CCSC in which patients received 2±1 Intravitreal injections of bevacizumab (2.5mg) on average during a follow-up of 24±14 weeks. Mean BCVA increased by 2±2 lines. Mean CRT improved significantly over follow-up (P<0.05), with six patients (50%) showing complete resolution of subretinal fluid. Above discussion shows that both anti VEGF, ranibizumab and bevacizumab are good options for those patients having macular edema particularly central serous chorio-retinopathy (CSC), not only acute but chronic central serous chorio-retinopathy (CCSC) can also be treated. Both are having their worth but in this study we used only ranibizumab to treat chronic central serous chorio-retinopathy and results were more than satisfactory.
It is still not clear that VEGF has some role in inducing central serous chorio-retinopathy but the use of anti VEGF for its treatment giving results. In our study, we used only one injection of ranibizumab 0.5mg/0.05ml and got excellent results in improving vision and reducing macular thickness with in the period of 15 days with no recurrence until now. So it is a good option to treat patients with central serous chorio-retinopathy particularly chronic central serous chorio-retinopathy without any damage to retinal or choroidal tissues with only one Intravitreal injection of ranibizumab 0.5mg/0.05ml.
I am thankful to Aftab Ahmed Khan, Atiqa Khan (final year BDS), Aisha Khan (4th year MBBS) students for their continuous help in compiling and editing data. Again Special thanks to my assistant Moin Shaikh for his help to keep OCT record and Jawaid Iqbal Memon for his support.
The authors declare there are no conflicts of interest.

©2017 khan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.