Advances in
eISSN: 2377-4290


Case Report Volume 6 Issue 5
1National Healthcare Group Eye Institute Tan Tock Seng Hospital Singapore
2Ophthalmology and Visual Sciences Khoo Teck Puat Hospital Singapore
3Department of Haematology Oncology National University Cancer Institute Singapore
4Department of Pathology National University Health System Hospital Singapore
Correspondence: Srinivasan Sanjay Ophthalmology and Visual Sciences Khoo Teck Puat Hopsital 90 Yishun Central 768828 Singapore, Tel +65 6555 8000
Received: March 08, 2017 | Published: April 11, 2017
Citation: Yee HY, Ming BCC, Chew YC, Cheong AWS, Putti TC, et al. (2017) An Ominous Red Eye. Adv Ophthalmol Vis Syst 6(5): 00195 DOI: 10.15406/aovs.2017.06.00195
A 49 year-old Malay gentleman presented with a history of a unilateral red eye of one week duration and slowly progressive blurring of vision over a year. On evaluation, he had unilateral anterior chamber inflammation, proptosis, ptosis, ophthalmoplegia and disc swelling. A diagnosis of orbital adnexal lymphoma with systemic nodal involvement and bone marrow infiltration was made following a series of investigations. He was treated systemically with eight cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) therapy and he managed to achieve complete remission. Our aim is to highlight that a unilateral painless red eye simulating conjunctivitis can be an ominous masquerade of orbital lymphoma, especially when there are associated orbital signs (proptosis, external ophthalmoplegia). A high index of suspicion is crucial as prompt diagnosis and timely management can achieve good outcomes.
Keywords: orbital lymphoma, uveitis, masquerade, proptosis, ophthalmoplegia
OAL, orbital adnexal lymphoma; R-CHOP, rituximab cyclophosphamide doxorubicin vincristine and prednisolone; LDH, lactate dehydrogenase; CT, computed tomography; PET, positron emission tomography; MALT, mucosa associated lymphoid tissue
Red eye is a common ocular presentation and is commonly treated in the primary care setting. Common causes include blepharitis, corneal abrasion, foreign body, subconjunctival hemorrhage, conjunctivitis and dry eye. Sight-threatening conditions causing unilateral red eye such as glaucoma, globe trauma, ocular or orbital infection or inflammation are often painful and accompanied by other discernible features and history. A detailed history taking of signs and symptoms coupled with a careful eye examination are hence crucial in differentiating the causes of the red eye. Our case demonstrates a painless unilateral red eye, which may appear benign as an early manifestation of a systemic and severe disease.
A 49 year-old Malay man presented to a general practitioner for redness of his left eye which was painless. He was given topical antibiotics but the eye redness persisted. He was subsequently referred to our ophthalmology department for further evaluation. His history revisited revealed that he also had blurry vision in the same eye progressively over a year. There was no tearing, discharge, photophobia or history of similar problem before. He was also systemically well. On examination, his best-corrected visual acuity for the affected left eye was 6/9 and was 6/6 on the right eye. He was also noticed to have left eye proptosis and a left sided partial ptosis (Figure 1a; photograph on presentation), which he had not noticed before. Left eye examination showed conjunctival injection, chemosis and anterior chamber inflammation; cells 3+and flare 2+(Standardization of Uveitis Nomenclature Classification). There were no signs of corneal oedema, fibrin or hypopyon. His optic nerve function was full but his left optic disc was slightly swollen with hyperaemia. There were no choroidal folds or obvious retinal or choroidal masses. His intraocular pressure was noted to be high at 31 mmHg in the left eye; as compared to 16 mmHg on the right. His left eye movements were also limited in all directions of gaze. Ophthalmic exam on the right was noted to be normal. Systemic examination discovered enlarged lymph nodes at the submandibular, sublingual and inguinal areas. His parotid gland was also enlarged.

Figure 1 (a) photograph taken pre-treatment showing left eye conjunctival injection and chemosis (b) photograph taken post-treatment showing complete resolution of conjunctival injection and chemosis in the left eye.
A series of investigations were soon conducted. An ultrasound B-scan (brightness scan) on his left eye showed absence of clinical sign of posterior scleritis (T- sign). Computed tomography scan of orbits and paranasal sinuses showed left proptosis and marked swelling of all the extra-ocular muscles in the left eye. There was no mass lesion seen. Blood tests were not conclusive but revealed high erythrocyte sedimentation rate (ESR) at 80 mm/hr and raised white cell count at 11.2 x 103/L with lymphocytosis at 51.5%. Thyroid function tests, haemoglobin and platelet levels were normal. The patient subsequently underwent left eye’s tenon capsule and superior rectus muscle biopsy for histological diagnosis. The superior rectus muscle histology displayed small B-cell lymphocytic infiltrate with irregular nuclei, indistinct nucleoli and scattered centroblasts (Figure 2; eye muscle histology). Immunohistochemistry showed a predominant CD20+B cell population in the biopsy sample. The tenon’s capsule biopsy showed perivascular lymphocytic

Figure 2 Eye muscle biopsy: Fibro-collagenous tissue with predominant atypical small ‘B’ cell lymphoid infiltrates and aggregates (Hematoxylin and Eosin, 40X).
Patient was co-managed with the department of haematology and oncology to rule out systemic involvement. Further biopsies were conducted and they took samples from his cervical lymph nodes and bone marrow. His lymph nodes had evidence of marginal zone B-cell lymphoma and suggestion of secondary nodal involvement of an extra-nodal mucosa-associated lymphoid tissue (MALT) lymphoma (Figure 3 and 4; lymph node histology). His right posterior superior iliac spine bone marrow biopsy was also consistent with B-cell lymphoma infiltrate. There were generalized lymphadenopathy from skull base to inguinal region together with bilateral renal hilar soft tissue infiltration noted on computed tomography of his chest, abdomen and pelvis consistent with the diagnosis of Non-Hodgkin lymphoma. An upper gastrointestinal tract endoscopy was also performed and revealed no gastrointestinal involvement.

Figure 3 Lymph node biopsy: High magnification image highlighting effaced nodal architecture. The predominant small ‘B’ lymphoid cells show monocytoid, centrocyte-like and few plasma cells (Hematoxylin and Eosin, 100X).
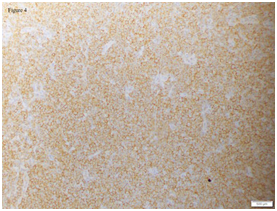
Figure 4 Lymph node biopsy: Immunohistochemical stain of lymph node shows diffuse immunoreactivity for ‘B’ cell marker (CD20, 40X).
In view of systemic multi-organ involvement of B-cell lymphoma, the patient was promptly started on chemotherapy with immunotherapy by the oncologist. He completed 8 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) therapy and managed to achieve complete remission. Upon review six months after his initial presentation, he was asymptomatic with left eye vision of 6/7.5 and a complete resolution of his left proptosis, lid ptosis and disc swelling (Figure 1b; photograph after treatment). In 2017, he has entered his 9 years after his first presentation and he has remained asymptomatic with no sign of relapse.
Orbital adnexal lymphomas (OAL) are the most common primary orbital malignancy in adults 60 years of age and older.1 Ocular adnexa comprise of orbit, extraocular muscles, conjunctiva, eyelids, lacrimal gland and apparatus and lymphomas of these structures account for approximately 1% to 2% of non-Hodgkin lymphomas and 8% of extranodal lymphomas.2 The majority of cases of OALs are primary extranodal lymphomas; however 10% to 32% are secondary tumours in patients with disseminated lymphomas.3-6 Ninety-five percent to 100% of reported cases of orbital adnexal lymphomas are B-cell neoplasms and majority are low grade with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) types being the most common histologic subtype constituting about 35% to 80% of cases.7–15
Orbital adnexa lymphomas (OAL) are mostly seen in the 5th to 7th decade of life (in Western and Japanese populations). Our case demonstrates a younger age of presentation for orbital adnexa lymphoma. This is consistent with a few studies in Korean population; which revealed a significantly younger age (median age ~46 years) at the time of diagnosis.15–17 Hence, a further study on our local population with OALs may be useful to better define the age demographics of these patients in Singapore. The risk factors for OAL remain unknown in the majority of cases but studies have shown associations with infections with Chlamydia psittaci.18 Chlamydia pneumoniae and Helicobacter pylori.19 A cohort study from the United Kingdom showed a significant prevalence of autoimmune thyrotoxicosis with thyroid orbitopathy preceding the diagnosis of OAL.20 Genetic abnormalities such as trisomy of chromosome 3 and chromosome 18 have been reported in ocular adnexal MALT lymphoma.21 Majority of cases of OALs are primary extranodal lymphomas, however patients with secondary tumours with disseminated tumours may present with initial ophthalmic symptoms. A high index of suspicion for systemic involvement is therefore required and thorough systemic enquiry and clinical examination, chest x-ray, serum lactate dehydrogenase (LDH), b2 microglobulin, chest x-ray, computed tomography (CT) of head and paranasal sinuses, chest, abdomen and pelvis, and bone marrow biopsy are usually performed upon diagnosis of OALs.
Some studies have suggested the use of positron emission tomography (PET) in detection of distant disease as it has higher sensitivity than CT scan in the detection of distant disease (86% vs 72%) in patients with OALs.22 The addition of PET imaging to CT and MRI has provided additional, clinically meaningful information that led to “upstaging” and change in patient management in 71% of cases.23,24 However, PET has low sensitivity (27%) in detecting orbital lesions.15 and the use of PET in OALs due to MALT lymphomas are questionable as overall fluorodeoxy glucose (FDG) uptake is reported in only 50% of cases of MALT lymphomas in other locations. Therefore, it may not be very useful in patients with MALT lymphomas.
Various treatment modalities are available for the management of patients with OALs including surgical resection, radiotherapy, chemotherapy and immunotherapy with monoclonal antibodies. Radiation therapy is the treatment of choice for the majority of patients with localized OALs whereas combined chemotherapy such as CHOP protocols are reserved for systemic and high grade lymphomas. Rituximab is a monoclonal chimeric anti-CD20 antibody and useful as a single-agent or combined with chemotherapy in treating both newly diagnosed and relapsed disease but early recurrence is common, particularly in pretreated disease.25 Our patient in this case is a good example that the treatment with systemic chemotherapy with R-CHOP therapy could potentially achieve complete remission with good prognosis.
A unilateral red eye could be an early ominous sign for an orbital lymphoma. A high index of suspicion is therefore required in diagnosing OALs especially when there are associated orbital signs (proptosis, external ophthalmoplegia). Early surgical biopsy and appropriate radio-imaging studies are essential for early and adequate diagnosis of orbital and systemic lymphoma. Radiation therapy is the treatment of choice for OALs and combined radio-chemotherapy is reserved for systemic and high-grade lymphomas.
None.
Dr Hah Yan Yee and Dr Srinivasan Sanjay had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author declares that there is no conflict of interest.

©2017 Yee, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.