Advances in
eISSN: 2573-2862


Research Article Volume 2 Issue 2
Department of Zoology, Utkal University, India
Correspondence: Abhimanyu Mohanta, Research Scholar, Post-Graduate Department of Zoology, Utkal University, Bhubaneshwar, Odisha, India- 751 004
Received: January 18, 2017 | Published: February 10, 2017
Citation: Mohanta A, Mohanty PK. Oral micronucleated cells-a cytodiagnostic approach. Adv Cytol Pathol. 2017;2(2):33–39. DOI: 10.15406/acp.2017.02.00013
Objective: Micronucleated cells (MNCs) are a special type of cytological atypia observed in the exfoliated cytosmears of control and oral neoplasm cases. Conflicting opinions related to the size of micronuclei (MNi) in oral epithelial cells prompted us to undertake for a complete resolution. Therefore, the objectives of the present study are to evaluate the cytopathogical importance of oral micronucleated cells (MNCs) and to bring about a comprehensive conflict resolution on account of the size of the micronucleus (MN) with respect to its parent nucleus (PN) through morphometric analysis.
Methodology: In a hospital-based Case-Control study, exfoliated cytosmears were collected from the age group, sex and oral sites- matched 272 subjects. Smears were fixed in 1:3 aceto alcohol. Out of two smearing slides, one was stained with Papanicolaou’s stain and the other was stained with Giemsa’s stain. Standard protocol was followed for scoring of MNCs. Cytomorphometry and photomicrography was done by using computer assisted Bio-Catalyst Cat Cam1.30microscope camera. Palaentological Statistics (PAST), Version 2.17.was used for statistical analysis.
Result: Significantly higher percentage (z<0.01) of pleomorphic MNCs were observed in cancer affected group than the normal counterpart. Nuclear-Cytoplasmic (N/C) ratios of the MNCs were found to be in increased state (1:12.5) in both sexes as compared to the normal ones (1:34.5 in male and 1:34.4 in female). Interestingly, the MN/PN ratios of the MNC were calculated to be 1:8.66 in male and 1:8.56 in female.
Conclusion: Due to universal occurrence, pleomorphism and increased nuclear-cytoplasmic (N/C) ratios as well as increased MN/PN ratios in both sexes, the MNCs are considered as a potential biomarker and a sensitive onco-indicator during oral carcinogenesis. Therefore, MNT is to be applied as a reliable cytogenetic tool for prognosis and diagnosis of human oral cancer.
Keywords: micronucleated cells, micronucleus, atypia, n/c ratio, mn/pn ratio
MNCs, micronucleated cells; MNi, micronuclei; N/C ratios, nuclear-cytoplasmic ratios; MN/PN ratios, ratio between area of micronucleus (MN) to the parent nucleus (PN)
Micronucleus (MN) is a microscopically visible, small, round or oval, non-refractive extra-nuclear cytoplasmic chromatin mass which consists of eccentric chromosomes or chromosomal fragments, originated either from aberrant mitosis or due to genotoxicity and completely detached from the parent nucleus (PN) of the respective somatic cell. MN is small, incomplete and originates either from the laggard chromosome or its fragment due to partial impairment of the spindle apparatus during anaphase. In the course of telophase, such chromatin material might be included into one or the other daughter cell, where it can either fuse with the main nucleus or form one or several secondary nuclei.1 The formation of MN in dividing cells is the result of chromosome breakage due to unrepaired or mis-repaired DNA lesions, or chromosome malsegregation due to mitotic malfunction. These events may be induced by oxidative stress, exposure to clastogens or aneugens, genetic defects in cell cycle checkpoint and/or DNA repair genes, as well as deficiencies in nutrients required as cofactors in DNA metabolism and chromosome segregation machinery.2
MN is surrounded by double membranes, and contains chromosomes or fragments of chromosomes which have not been incorporated into one of the daughter nuclei during cell division.3,4 Conflicting opinions related to the size of micronuclei (MNi) still exist in literature. The relative sizes of the MN are reported to be approximately 1/5th to 1/3rd area of the PN.
The cell bearing one or multiple number of micronuclei (MNi) is known as micronucleated cell (MNC). Appearance of MNCs due to potential carcinogens and environmental mutagens has been shown to be a reliable and sensitive biomarker for cytogenetic damage.5 Among a number of tests, the micronucleus test (MNT) has been implicated both for in vivo and in vitro cytogenetic analysis to indicate chromosomal aberrations.6,7 Application of the MNT in exfoliated buccal cells is an innovative genotoxicity technique, which holds promise for the study of epithelial carcinogens. Micronuclei are suitable internal dosimeters for revealing tissue-specific genotoxic damage in individuals exposed to carcinogenic mixtures.8-10 Almost all the papers published so far are based on either mutagenicity or carcinogenicity of MNCs and none of these was reported on its pathogenicity. Therefore, the objectives of the present study are to evaluate the frequency and pathogenicity of such oral MNCs with respect to age, sex and site through cytodiagnostic approach and to bring about a comprehensive conflict resolution on size of the MNi in oral MNCs through morphometric analysis.
The subjects
In a hospital-based Case-Control study, 136 oral neoplasm cases of which 82 (60.3 %) were male and 54 (39.7 %) were female. The female to male proportion was 1: 1.56. Detailed case-history was collected and written consent was also obtained from each patient individually. All the patients were referral patients coming from different regions of Odisha State and were registered at the Out-patient Department (OPD) of Acharya Harihar Regional Cancer Centre (AHRCC), Cuttack, Odisha, India- the only Government Hospital of the Odisha State- dedicated for the treatment of cancer patients, during May 2007 to May 2009. The patients included in this study had neither undergone radio-therapy nor chemotherapy at all. Age group, oral site and sex-matched a parallel set of 136 normal healthy individuals were also included as Control group, Thus, a total of 272 subjects were included in this study.
Oral sites
According to International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), six oral sites, such as lips (C00), tongue (dorsal- C01, ventral-C02), alveolus and gingiva (C03), floor of the mouth (C04), palate (C05) and buccal (cheek) mucosa (C06) were taken into account for this study. On the basis of ICD-10, out of 136 oral cancer cases, 5 (6.1%) males and 6 (11.1%) females were reported to have lip cancer, 11 (13.4%) males and 7 (12.9%) females were of tongue/ lingual neoplasm, 16 (19.5%) males and 6 (11.1%) females were of cancer at alveolus and gingiva, 7 (8.5%) males and 6 (11.1%) females were affected at floor of the mouth, 6 (7.3%) males and 3 (5.6%) females were of palatal neoplasm, and 37 (45.2%) males and 26 (48.2%) females were suffering from cancer at buccal mucosa.
Pathogenicity: Based on the gross clinical study, out of 136 oral cases, 15 (18.3%) males and 14 (25.92%) females were of leucoplakia, 17 (20.73%) males and 9 (16.7%) females were of erythroplakia, 35 (42.7%) males and 20 (37.0%) females were of benign cases and 15 (18.3%) males and 11 (20.4%) females were of malignant ones.
Age group: The recorded age of the patients varied from 30-87 years with an average of 53.33±12.11 year. Therefore, the collected samples were categorized into three broad age-groups such as 30-49, 50-69, and 70-89 years.
Addiction: A total of 126 (92.6%) cases were addicted to different forms of tobacco and alcohol for more than 15 years and 10 (7.4%) were non-addicted.
Collection of samples fixing and staining
Prior to the collection of samples, the subjects were asked to rinse their mouth with normal fresh water. Exfoliated scrape cytosmears were collected over the precleaned-coded glass-slides. Utmost care was taken for uniform spreading of the oral smear while smearing. Exfoliated cytosmears were fixed in 1:3 aceto-alcohol (1 part of glacial acetic acid and 3 part of absolute ethyl alcohol). Two slides were prepared from the respective oral sites (ICD-10), of which one slide was stained with Papanicolaou’s stain and the other was stained with Giemsa’s stain.
Scoring morphometry and statistical analysis
Stained slides were observed under Hunds-H500 binocular light microscope. Suitable MNCs were scored as per the standard scoring protocol.11-13 Cytomorphometry was done and photomicrographs were taken out by using computer assisted Bio-Catalyst Cat Cam 1.30microscope camera. Palaentological Statistics (PAST®), Version 2.17.was used for statistical analysis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Subject Research Committee (SRC) of Utkal University (Reference No EC/UU-38832/2007), Bhubaneshwar, Odisha, India and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Micronuclei were detected in the exfoliated oral sqamous cells scraped from different sites of the oral cavity of the normal and cancer affected individuals. These were observed to be microscopic, round or oval body and as small as 1/8.6th of the parent nucleus (PN). The cell bearing one or multiple number of micronuclei (MNi) is known as micronucleated cell (MNC). The MNCs are unique type of cytological atypia found to contain either a single MN or more number of MNi within a cell. Site specific and age group-wise enumeration of MNCs and their morphometry exhibit the degree of pathogenicity in the oral squamous cells of the control and cancer affected individuals which are described as under.
Site-specific scoring of MNCs
In Control group, the highest percentage (0.143%) of MNCs scored from the buccal mucosa followed by tongue, floor of the mouth and palate- 0.10% in each in the age group of 70-89 years and lowest (0.0125%) in alveolus and gingival in the age group of 50-69 years among male individuals. In case of female, the highest percentage (0.125%) of MNCs was recorded from buccal mucosa in the age group of 30-49 years and the lowest (0.025%) was scored at lip and lingual sample in the age group of 50-69 years respectively. However, the mean percentage was calculated to be the highest in lip in both sexes (male-0.04%, female-0.166%) and the lowest one at alveolus and gingiva in male (0.013%) and at floor of the mouth in female (0.01%). A total of 22 (0.026%) MNCs from 82 normal male and 11 (0.020%) MNCs from 54 female were scored from all sites of the normal individuals.
In case of cancer affected group, the frequency of MNCs was recorded to be highest (5.1%) among the lip neoplasm cases in the age group of 70-89 years and the lowest (0.828%) was scored from buccal mucosa sample in the age group of 30-49 years in male. In case of female, the highest percentage (4.5%) of MNCs was recorded from the floor of the mouth in the age group of 50-69 years and the lowest frequency (0.40%) was found at alveolus and gingiva in the age group of 30-49 years. Furthermore, the mean percentage was recorded to be the highest in male (3.7%) and female (2.86%) among the lip neoplasm cases. However, lowest frequency was calculated to be 0.60% in both sexes among palatal neoplasm cases. In to, 1234 (1.504%) MNCs from 84 male cases and 770 (1.425%) MNCs from 54 female cases were scored from all sites of the cancer affected individuals (Figure 1). It is important to note that in some normal healthy individuals, MNCs were not found at all. Contrary to that, pleomorphic MNCs were observed in all the samples collected from the different sites of the oral cancer cases. From the test of proportion, the z-values were also found to be greater than the normal distribution (z=2.576) and so, the obtained results are highly significant at 1% level of confidence (Table 1).
Sites |
Age Group |
Control Group |
Affected Group |
Z-Value* |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
No of Samples |
MNC Scored |
Mean Percentage |
No of Samples |
MNC Scored |
Mean Percentage |
||||||||||
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
M |
F |
||
Lip |
30-49 |
2 |
Nil |
1 |
Nil |
0.04 |
0.166 |
2 |
Nil |
58 |
Nil |
4 |
2.86 |
4.333* |
12.162* |
50-69 |
2 |
4 |
1 |
1 |
2 |
4 |
76 |
98 |
|||||||
70-89 |
1 |
2 |
Nil |
Nil |
1 |
2 |
51 |
74 |
|||||||
Tongue |
30-49 |
5 |
2 |
1 |
1 |
0.027 |
0.028 |
5 |
2 |
62 |
32 |
2 |
1.87 |
13.718* |
11.608* |
50-69 |
5 |
4 |
1 |
1 |
5 |
4 |
104 |
84 |
|||||||
70-89 |
1 |
1 |
1 |
Nil |
1 |
1 |
28 |
22 |
|||||||
Alveolus and gingiva |
30-49 |
4 |
3 |
Nil |
Nil |
0.013 |
0.016 |
4 |
3 |
24 |
12 |
1 |
0.566 |
10.773* |
5.590* |
50-69 |
8 |
3 |
1 |
1 |
8 |
3 |
78 |
22 |
|||||||
70-89 |
4 |
Nil |
1 |
Nil |
4 |
Nil |
64 |
Nil |
|||||||
Floor of the mouth |
30-49 |
4 |
1 |
Nil |
Nil |
0.028 |
0.1 |
4 |
1 |
66 |
8 |
3 |
1.26 |
13.171* |
7.750* |
50-69 |
2 |
4 |
1 |
2 |
2 |
4 |
54 |
45 |
|||||||
70-89 |
1 |
1 |
1 |
1 |
1 |
1 |
55 |
23 |
|||||||
Palate |
30-49 |
4 |
1 |
Nil |
Nil |
0.033 |
Nil |
4 |
1 |
18 |
4 |
1 |
0.6 |
5.536* |
6.018* |
50-69 |
1 |
2 |
1 |
Nil |
1 |
2 |
8 |
14 |
|||||||
70-89 |
1 |
Nil |
1 |
Nil |
1 |
Nil |
10 |
Nil |
|||||||
Buccal mucosa |
30-49 |
# |
4 |
5 |
1 |
0.029 |
0.015 |
14 |
4 |
## |
26 |
1 |
1.27 |
21.25 |
17.964 |
50-69 |
# |
19 |
4 |
2 |
16 |
19 |
## |
## |
|||||||
70-89 |
7 |
3 |
2 |
1 |
7 |
3 |
## |
49 |
|||||||
All sites |
30-89 |
# |
54 |
22 |
11 |
0.026 |
0.02 |
82 |
54 |
## |
## |
2 |
1.425 |
34.472* |
26.353* |
Table 1 Oral site, age group and sex-wise enumeration and analysis of MNCs in control and cancer affected group
Cytomorphology of the MNCs
Pleomorphism was well observed among the MNCs in all sites and at different stages of oral carcinogenesis. Morphologically, the MNCs were observed to be either well differentiated squamous cell (WDSC) or moderately differentiated squamous cell (MDSC) type. The well differentiated MNCs were having a close resemblance with the normal oral squamous cells (NOSCs) having more or less polyhedral, with well defined cell boundary and distinct round or oval nucleus. On differential staining, the NOSCs were appeared to be sky-blue color in Papanicolaou’s stain and magenta color in Giemsa’s stain respectively (Figure 1). These well differentiated MNCs were restricted to precancerous lesions whereas the moderately differentiated MNCs were observed in benign and malignant oral neoplasms and mostly resembled with keratinized spindle cells (KSCs), keratinized tadpole cells (KTCs) and keratinized round cells (KRCs) (Figure 2). So far as frequency of MNi is concerned, one to multiple numbers of micronuclei was observed within an MNC (Figure 3). Cytoplasm of the MNC was found to be either hypokeratinized (in leukoplakia) or hyperkeratinized (in erythroplakia, benign and malignant neoplasm cases). In other words, intensity of keratin expression is directly related to the degree of pathogenicity during multistep oral carcinogenesis.
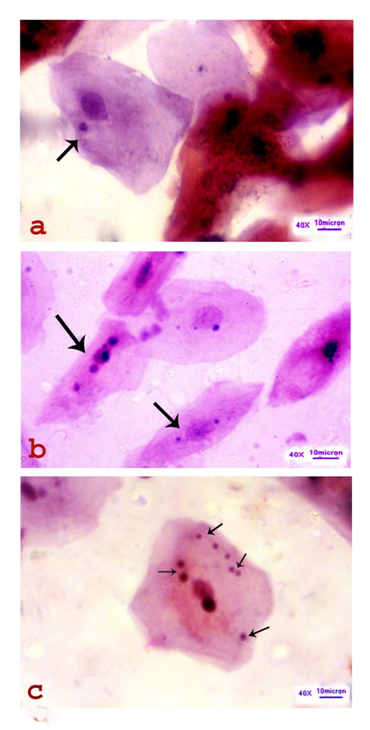
Cytomorphometry of the NOSCs, MNCs and MNI
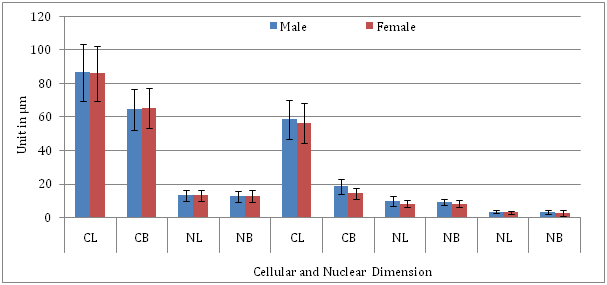
Cytometric analysis (Table 2) showed that the mean length and breadth of the normal oral squamous cells (NOSCs) in males were 86.50µm (±17.234µm) and 64.23µm (±12.125µm) respectively having an area of 5555.89µm2 (±208.362µm2). The nucleus of the normal squamous cell in male was measured to be 12.95µm (+3.368µm) in length and 12.43µm (±3.235µm) in breadth with an area of 160.968 µm2 (±10.895µm2). The nuclear-cytoplasmic (N/C) ratio in male was calculated to be 1:34.5. In case of females, the mean length, breadth and area of the normal oral squamous cells were 85.875µm (±16.374µm), 65.012µm (±11.876µm) and 5582.905µm2 (±194.457µm2) respectively. The mean length and breadth of the nucleus were 12.920µm (±3.544µm) and 12.530µm (±3.452µm). Thus, the mean nuclear area of the normal squamous cells in females was found to be 161.887µm2 (±12.233µm2). Hence, the nuclear-cytoplasmic (N/C) ratio was calculated to be 1:34.4 in females.
Sex |
Cell type |
No of cells scored |
Cytoplasm (C) |
Nucleus (N) |
N/C Ratio (MN/PNRRatio*) |
||||
|---|---|---|---|---|---|---|---|---|---|
Mean length |
Mean breadth |
Mean area |
Mean length |
Mean breadth |
Mean area in mm2+SD |
||||
Male |
NOSC |
1000 |
86.50 ±17.234 |
64.23±12.125 |
5555.89±208.362 |
12.95±3.368 |
12.43±3.235 |
160.968±10.895 |
01:34.5 |
MNC |
1234 |
58.552±11.725 |
18.389±4.370 |
1076.712±51.238 |
9.583±3.01 |
8.976±2.02 |
86.017±6.080 |
01:12.5 |
|
MN |
2042 |
58.552±11.725 |
18.389±4.370 |
1076.712±51.238 |
3.182±1.031 |
3.12±1.120 |
9.928±1.155 |
1:108.5(1:8.66*) |
|
Female |
NOSC |
1000 |
85.875±16.374 |
65.012±11.876 |
5582.905±194.457 |
12.92±3.544 |
12.53±3.452 |
161.887±12.233 |
01:34.4 |
MNC |
770 |
56.231±12.187 |
14.178±3.223 |
797.243±39.278 |
8.058±2.214 |
7.875±2.032 |
63.456±4.098 |
01:12.5 |
|
MN |
1855 |
56.231±12.187 |
14.178±3.223 |
797.243±39.278 |
2.824±1.085 |
2.617±1.788 |
7.39±1.939 |
1:107.8(1:8.58*) |
|
Table 2 Sex-wise cytomorphometric analysis of NOSC, MNC and MN
*MN/PN Ratio, SD- Standard Deviation MNC- Micronucleated cells, MN-Micronucleus, NOSC-Normal oral squamous cell
Cytometrically, the mean length, breadth and area of the MNC in male were found to be 58.552mm (±11.725mm), 18.389mm (±4.370mm) and 1076.712mm2 (±51.238mm2), while, the mean length breadth and area of the nucleus were recorded to be 9.583mm (±3.01mm), 8.976mm (±2.02mm) and 86.017mm2 (±6.080mm2) respectively. Hence, the N/C ratio was calculated in MNC as 1:12.5.in male. In case of females, the length, breadth and area of the MNC was measured to be 56.231mm (±12.187mm), 14.178mm (±3.223mm) and 797.243mm2 (±39.278mm2), while, the nuclear length, breadth and area were calculated to be 8.058mm (±2.214mm), 7.875mm (±2.032mm) and 63.456mm2 (±4.098mm2) respectively (Figure 4). The N/C ratio of the MNC was found to be 1:12.5, which was equal to that of female.
An MNC was found to be either mono-, bi-, tri- or multi-micronucleated. A total of 2042 micronuclei from 1234 MNCs and 1855 MNi from 770 MNCs were scored from the male and female cases respectively. The mean area of the MN was calculated to be 9.928mm2 (±1.155Imm2) in male and 7.390mm2 (±1.939mm2) in female. Although, MN/C ratio was found to be 1:108.5, the MN/PN ratio was calculated to be 1:8.66 in male. Similarly, the MN/C ratio and MN/PN ratio were found to be 1:107.8 and 1:8.56 respectively, in female (Figure 5).
Cytological pleomorphism is a unique feature in the exfoliated cytosmear scraped from the human oral neoplasms. Out of a number of typically atypical detectable atypias, oral micronucleated cell (MNC) is one of those cytological atypia has some essential cytodiagnostic features which prompted us to analyse in detail.
Study on MN and MNC is not new in biomedical science. MN was previously known as ‘Howell-Jolly body’ in medical science. Due to its association with chromosomal aberrations, MN has been used since 1937 as an indicator of genotoxic exposure.14 Since, the formation of micronuclei in the eukaryotic cells was an end point of chromosomal damage or segregation errors, the presence of MNi reflects a genotoxic or carcinogenic exposure .15 The assay was reliable and technically easy to perform. The direct correlation between the MNi formation and genomic damage make the MN assay an efficient alteration to the metaphase analysis.16
MN is an abnormal product of genotoxic and carcinogenic effect on oral mucosal cells. And so, the oral MNCs behave abnormally, become uncontrolled, go beyond the cell cycle and initiate oral carcinogenesis. Similar to other types of malignant neoplasia, oral cancer results from alterations (point mutations and chromosomal abnormalities) in genes that control the cell cycle, and/or in genes that are involved in DNA repair. In addition to the potential for metastasis, cancer is characterized by the loss of the ability of cells to evolve to death when genetic damage occurs (apoptosis).17,18 Therefore, MNCs are identified as a potential biomarker and a sensitive onco-indicator in Cytogenetics and micronucleus test (MNT) has been accepted as an ideal in vivo cytogenetic screening procedure for the detection of induced structural chromosome aberrations in general and early detection of oral cancer, in particular.19
The MNT, developed by Heddle20 and Schmid,21 is an in vivo and in vitro short-term screening method which has been widely used to detect genotoxic effects.22 It is one of the simplest, reliable, least expensive and rapid screening system for both clastogenic (chromosome breakage and formation of acentric fragments) and aneugenic (chromosome lagging and effects on spindle) effects.23 Clastogenic and aneugenic agents are known to affect the spindle apparatus, and can be differentiated on the basis of the relative induced micronucleus sizes or in the presence of kinetochores.24,25 It is evident that micronuclei originating from chromosomal fragments are generally ought to be smaller than those resulting from whole chromosome(s). Due to aneugenic effect, bigger MNi are formed from the damage to the spindle apparatus of the cell resulting in the exclusion of whole chromosome, whereas clastogenic effect leads to the formation of smaller MNi from structural aberrations causing fragments of chromosomes.26,27 Pampalona et al.28 have reported that whole chromosome loss is promoted by telomere dysfunction in primary cells.28
Although, MNT was originally employed in testing of bone narrow, now it has been applied for many purposes including the testing of the carcinogenic pathogenicity of the oral cavity.29 The MN test on human exfoliated cells provides the evidence of exposure to carcinogens and measures the degree of carcinogenic exposure in the tissue from which cancer develops. Increase in MNi frequency in buccal mucosa of tobacco and alcohol users indicates a high risk group for oral cancer.30,34 Mohanta et al. [35] have reported that genotoxicity of tobacco and alcohol triggers the induction of MNi and MNCs in the oral mucosa and their combined effect was found to be more genotoxicity than the single use on the buccal mucosal cells. The formation of more number of MNCs in chewer-smoker-alcoholics group followed by in chewer-smokers than single addicted (chewers, smokers and alcoholics) groups has proved the potentiality of genotoxic effect of tobacco and alcohol on oral mucosal cells. The results also support the facts of earlier findings that longer the addiction, more the number of MNi formations in oral mucosal cells.35
Bansal et al.36 have opined that people who are exposed to organic solvents, anti-neoplastic drugs, polycyclic aromatic hydrocarbons, drinking water contaminated with arsenic and paints with lead content shows significantly higher frequencies of MNi in exfoliated mucosal cells.36 In real sense, adopting modern lifestyle like chewing and smoking of tobacco as well as drinking of alcohol, dependency on fast food, diet, especially vitamin deficiencies and supplementation are also associated with the genetic damage followed by MNi formation in the oral mucosa.37
Occurrence of MNCs was observed in the exfoliated oral smears of both control and cancer affected groups. Pertaining to the scoring of micronucleus, the scored elements were MNCs and not the number of micronuclei.1,21,38 In the present study, the frequency of MNCs was recorded to be highest (5.1%) among the lip neoplasm cases in the age group of 70-89 years and the lowest (0.828%) was scored from buccal mucosa sample in the age group of 30-49 years in male. In case of female, the highest percentage (4.5%) of MNCs was recorded from the floor of the mouth in the age group of 50-69 years and the lowest frequency (0.40%) was found at alveolus and gingiva in the age group of 30-49 years. This indicates that frequency of MNCs increases with increase of age in the respective oral sites. Probable cause may be attributed to the longer period of consumption of tobacco and alcohol as well as exposure to various carcinogenic and mutagenic agents in day to day life. Furthermore, the mean percentage was recorded to be the highest in male (3.7%) and female (2.86%) among the lip neoplasm cases and the lowest frequency was calculated to be 0.60% in both sexes among palatal neoplasm cases, in the present study. Possibly, longer exposure to the solar ultraviolet light and various environmental carcinogens, epithelial cells of the lip get mutated and enhance the MNi formation. On the other hand, palate is the only site of the oral cavity, which is least affected by various carcinogens except smoke. Therefore, the mean percentages of MNCs were found to be the highest in lip and lowest in palatal neoplasm cases.
To our best of knowledge, none of the published papers has reported on the cytopathological aspect of MNCs. Mostly, these were devoted to analyse their frequencies in different conditions and stages of oral carcinogenesis. Furthermore, age group, sex and oral site-specific analysis on MNCs has also been ignored. However, the present study has fulfilled that gap. Due to pleomorphism, the MNCs exhibited a greater degree of cellular differentiation. Morphologically, the MNCs were observed to be either WDSC or MDSC type. The well differentiated MNCs had a close resemblance with the NOSCs and were restricted to precancerous lesions whereas the moderately differentiated MNCs were observed in benign and malignant oral neoplasms and mostly resembled with the well defined KSCs, KTCs and KRCs. Morphometrically, the N/C ratio of the MNC was found to be 1:12.5 in both sexes which was observed to be in an increased state and more than those of normal counterparts (1:34.5 in male and 1: 34.4 in female).
On account of the size of the MN, ‘doctors differ’. Conflicting and contradictory opinions still exist, so far as the size of the MN is concerned. Authentic and reliable morphometrical data are not found in the published literature. In his scoring criteria, Schmid38 has mentioned that the size of the MN was approximately 1/5th of nucleus of the concerned cell.1 On the other hand, many authors accepted its size to be less than 1/3rd area of the PN.5,11,13 In the present study, cytometrically, the MN/ PN ratios were calculated to be 1:8.66 in male and 1:8.56 in female which not only contradicts the earlier findings but also able to bring about an authentic conflict resolution in the field of cytopathology.
Oral MNCs are either aneugenic or clastogenic product of genotoxic and carcinogenic effect on oral mucosal cells. Pleomorphic oral MNCs are observed to be either hypokeratinized (in leukoplakia) or hyperkeratinized (in erythroplakia, benign and malignant neoplasm cases). Intensity of keratin expression is directly related to the degree of pathogenicity during multistep oral carcinogenesis. The frequencies of MNi and MNCs are also found to be in an increasing trend in respective oral sites with increasing age and exposure to various carcinogenic agents in both sexes. Morphometrically, the N/C ratio of the MNC was found to be 1:12.5 in both sexes which was observed to be in an increased state and more than those of normal counterparts (1:34.5 in male and 1: 34.4 in female) indicates the tendency towards malignancy. Particularly, occurrence of moderately differentiated MNCs even in premalignant lesions at any site may be accepted as a potentially malignant one. In the present study, the MN/ PN ratios were calculated to be 1:8.66 in male and 1:8.56 in female which not only contradicts the earlier findings but also able to bring about an authentic conflict resolution in the arena of cytopathology. Therefore, cytodiagnostically, MNC is really a potential biomarker as well as a sensitive onco-indicator and MNT is considered as a novel cytogenetic tool for prognosis and diagnosis of human oral cancer.
The authors are thankful to Prof. Gadadhar Parida, M.D, formerly Professor & Head, Department of Oncopathology, Acharya Harihar Regional Cancer Centre (AHRCC), Cuttack, Odisha, India for his guidance and supervision during cytopathological analysis. We are also indebted to the Head P.G. Dept. of Zoology, Utkal University, Vani Vihar, Bhubaneshwar, Odisha, India and to the Director, AHRCC, Cuttack, Odisha, for permitting us to collect samples from oral cancer patients and also for providing library and laboratory facilities. One of us (AM) is grateful to the University Grants Commission (UGC), New Delhi, India for awarding UGC Meritorious Research Fellowship to carry out the research work.
The author declares no conflict of interest.

©2017 Mohanta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.