Advances in
eISSN: 2573-2862


Mini Review Volume 4 Issue 1
Department of Biology, Federal University of Espirito Santo– Center for Natural, Exact and Health Sciences, Brazil
Correspondence: Victor Ventura de Souza, Department of Biology, Federal University of Espirito Santo–Center for Natural, Exact and Health Sciences, Brazil
Received: April 13, 2017 | Published: February 27, 2019
Citation: Spadeto MS, Souza VV. Induction of apoptosis from exposure to aristolochic acid. Adv Cytol Pathol. 2019;4(1):15-16. DOI: 10.15406/acp.2019.04.00073
The genus Aristolochia gathers plants used in folk medicine, which present in their composition the Aristolochic acids I and II. Among the major toxicological events caused by Aristolochic acids is programmed cell death. Aristolochic acid induced apoptosis occurs in target organs such as the liver, kidney, pancreas, testis, ovary, intestine and lung. Although it is beneficial for the cure of various diseases Aristolochias or Aristolochic acids I and II can also cause necrosis. The objective of this work was to compile information about the effects of Aristolochic acids on the induction of apoptosis. The present work makes an alert when consuming this herb, and derivatives that possess Aristolochic acids.
Keywords: Aristolochia, cell death, gene synthesis, caspase 3
The Aristolochic acids (AAs) I and II are present in all plants of the family Aristolochiaceae, commonly used to aid in weight loss and the cure of rheumatism, arthritis, arthrosis, reduction bacterial infection and of malarial fever.1 On the other hand, the AAs are considered apoptosis inducers mainly in renal cells, the target organ of AAs.2 Apoptosis is a physiological event that occurs naturally in all organisms. However, when it is grossly induced, it causes irreversible damage. The cells that are entering into apoptosis have a characteristic morphology with condensation of the genetic material followed by fragmentation of the nucleus (Figure 1).3,4
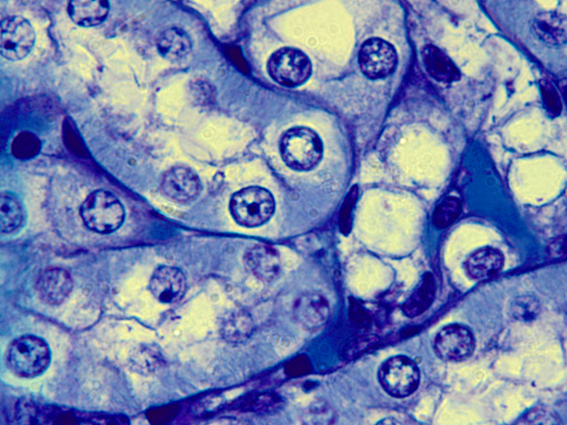
Figure 1 Apoptosis induced by Aristolochic acid. Note some cells with condensed nuclei are the first step for the cell to enter into apoptosis. Repair the fragmentation of the characteristic genetic material in apoptotic cells. 1000xmagnification. source: personal archive.
The process of renal lesion occurs in 4 stages:5 The first step is the loss of cell adhesion, releasing the cells from the cytoskeleton; The vimentin, an important component of the cytoskeleton, is no longer expressed; Subsequently, cell membrane disruption occurs and, finally, Migration of cells. These four events cause oxidative stress inducing apoptosis.
AAs and their derivatives are extremely toxic to LLC–PK1 cells, inducing injuries, which stimulate the synthesis of Caspase 3 and 7, leading to apoptosis, regardless of dosage.6 AAs strongly interfere with gene expression, stimulating the expression of genes related to apoptosis, cell cycle regulation, nuclear organization and mitochondrial transport. Consequently, apoptosis inducing proteins such as BAX (BLC–2–associated protein), BCL2L2, BNIP3, CASP3, CCL2, NAIP (protein inhibitor), PRKCA (protein kinase C), TNFRSF1B, TNFRSF21 and TNFSF6 are expressed.7 AAs can also induce apoptosis from mitochondrial stress, causing loss of membrane permeability, thus releasing cytochrome C.8 Apoptosis was verified by different pathways: activation of ERK 1/2, synthesis and activation of caspase 3, increased synthesis of Bax protein (pro–apoptotic protein) and decline in Bcl–2 (anti–apoptotic) synthesis, Apoptotic control. On the other hand it was observed that the synthesis of the Blc–2 protein was reduced drastically while the Bax protein had its synthesis attenuated.9 This decrease in protein synthesis can be attributed to the presence of AAs. The AAI induces apoptosis via ERK 1/2 in addition to the activation of the p38 protein, thus concluding that AAI–induced apoptosis is correlated with loss of glutathione (GSH).10
Mitochondria were isolated from HK–2 renal cells in order to ascertain the potential of AAI in mitochondrial membrane permeability. The AAI at the 50μm dosage along with 20μm Ca2+ for 24 h resulted in mitochondrial swelling, Ca2+ extrapolation, membrane depolarization and cytochrome C outflow, decreased ATP production and high activity of caspase 3, pro–apoptotic protein.11 Other authors using the same methodology and cell line, but with 100μm of AAs induced stress and increased concentration of intracellular Ca2+ and changes in mitochondria. Thus, they concluded that the toxicological and cytotoxic effect of AAs is potentiated when the concentration of Ca2+ increases.12 Corroborating with this information, an experiment with HK–2 renal cells showed that Ca2+ potentiates the effect of AAI on mitochondria.12 In addition, it has been shown that AAs also cause oxidative stress in the endoplasmic reticulum releasing calcium into the intracellular environment, so calcium accumulation leads to mitochondrial stress and consequently apoptosis.13
AAs are potent inducers of apoptosis, leading to cell death on a large scale. We do not recommend the use of any herb of the family Aristolochiaceae and no product containing the AAs.
None.
The authors declare there is no conflict of interest.

©2019 Spadeto, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.