Advances in
eISSN: 2573-2862


Research Article Volume 2 Issue 1
Department of Pathology and Laboratory Medicine, Cornell University, USA
Correspondence: Rhonda K Yantiss, Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, 525 East 68th Street, New York, NY 10065, USA, Tel 212-746-2824, Fax 212-746-8624
Received: October 30, 2016 | Published: February 9, 2017
Citation: Saab J, Estrella JS, Chen YT, et al.Immunohistochemical and molecular features of gastric hyperplastic polyps. Adv Cytol Pathol. 2017;2(1):25–31. DOI: 10.15406/acp.2017.02.00012
Context: Gastric hyperplastic polyps are generally considered to be inflammatory lesions that develop in association with gastritis. Large polyps may contain foci of dysplasia, suggesting that they represent neoplasms, although molecular data regarding their pathogenesis are lacking.
Objective: To describe the clinical, pathologic, and molecular features of large (>1cm) gastric hyperplastic polyps and compare them to those of small (=1cm) hyperplastic polyps and gastric adenomas.
Design: 15 large hyperplastic polyps (>1cm), including 4 with dysplastic foci, 10 small hyperplastic polyps (=1cm), and 9 gastric adenomas were evaluated for immunohistochemical expression of MGMT, MLH-1, ß-catenin, p27, p16, and p53. Polyps with dysplasia or abnormal ß-cateninexpression were assessed for APC and ß-catenin (CTNNB1) mutations. Large hyperplastic polyps and adenomas were analyzed for KRAS and BRAF mutations.
Results: Complete loss of MGMT expression was significantly more frequent in large hyperplastic polyps (47%), often in combination with loss of p27 (47%), compared to adenomas (0% and 0%, p=0.02, respectively) and smaller lesions [0% (p=0.02) and 10% (p=0.09), respectively]. All non-dysplastic hyperplastic polyps showed preserved MLH1 and lacked nuclear ß-catenin staining, but 3 with dysplasia had APC or ß-catenin mutations. KRAS or BRAF mutations were detected in 20% of large hyperplastic polyps and 11% of adenomas. Most polyps were entirely negative for p16, although 6 (40%) large hyperplastic polyps and 3 (33%) adenomas showed expression in <10% of the epithelium. None of the polyps showed >10% p53 expression in the epithelium.
Conclusion: Small gastric hyperplastic polyps are likely non-neoplastic, reparative lesions, but large polyps often show immunohistochemical and molecular abnormalities such as concomitant loss of MGMT with p27 staining, APC or ß-cateninmutations in areas of dysplasia, and KRAS or BRAF mutations. These findings raise the possibility that some large hyperplastic polyps of the gastric mucosa represent early neoplasms.
Keywords: stomach, serrated polyp, adenoma, dysplasia, mgmt, ß catenin, microsatellite instability, KRAS, BRAF
Gastric hyperplastic polyps are common epithelial polyps of the stomach encountered in 0.4-1.0% of upper endoscopic examinations.1,2 Together with fundic gland polyps, these lesions account for approximately 80% of all gastric polyps.1 Most gastric hyperplastic polyps show regenerative epithelial changes, characterized by architecturally distorted, cystically dilated glands lined by foveolar-type epithelial cells, elongated pits and edematous inflamed lamina propria. Lesional epithelial cells may contain voluminous mucinous cytoplasm or show mucin depletion, but cytologic atypia is minimal or mild.3 Foci of intestinal metaplasia are present in about 16% of cases. Most gastric hyperplastic polyps are solitary sessile lesions that span <1 cm. They show a predilection for the antrum, although polyps also occur in the cardia near the gastro esophageal junction, in the body, or at gastroenteric anastomoses. Twenty percent of affected patients have multiple polyps, especially those with autoimmune gastritis.1,4 These lesions have been traditionally considered to be post-inflammatory regenerative polyps due to their frequent association with mucosal inflammation: 85% are associated with some type of gastric injury, including autoimmune gastritis, Helicobacter pylori (H. pylori) infection, prior surgical anastomosis, and chemical gastropathy.4
Approximately one-third of hyperplastic polyps measure 1 cm or more in diameter, and some contain foci of dysplasia or carcinoma.4 The occurrence of dysplasia in these large lesions is difficult to reconcile with the concept of a post-inflammatory/ reparative polyp and raises the possibility that some large gastric hyperplastic polyps are neoplasms, similar to large serrated polyps of the colon, previously considered to be hyperplastic polyps.3-8 However, in contrast to colorectal hyperplastic polyps, which have been extensively studied and shown to harbor frequent BRAF or KRAS mutations, the immunohistochemical and molecular features of gastric hyperplastic polyps have not been systematically evaluated, and their relationship to dysplastic gastric polyps (adenomas) is largely unknown.9-11 Therefore, the aim of this study is to describe the clinical, pathologic, and molecular features of large (>1 cm) gastric hyperplastic polyps and compare them to those of small (=1 cm) hyperplastic polyps and gastric adenomas. We utilized a panel of immunohistochemical markers [O6-methylguanine DNA methyltransferase (MGMT), MLH1, p27, p16, p53, and ß catenin] and performed molecular analyses to assess these lesions for alterations affecting the Wnt signaling and mitogen activated protein (MAP) kinase (KRAS and BRAF) pathways, in order to gain insight into their pathogenesis.
Clinical features
The files of the Department of Pathology and Laboratory Medicine were searched for gastric hyperplastic polyps and gastric adenomas. Exclusion criteria included an underlying polyposis disorder (PTEN-hamartoma tumor syndrome, juvenile polyposis, familial adenomatous polyposis, and Cronkhite-Canada syndrome) and Menetrier disease. We selected 34 gastric polyps for evaluation, including a study group of 15 hyperplastic polyps that measured at least 1 cm in diameter and 19 controls (10 hyperplastic polyps measuring less than 1 cm and nine adenomas). Information regarding patient age, gender, polyp size and location, prior or concomitant chronic gastritis and/or H. pylori infection, autoimmune gastritis, chemical gastropathy, and prior gastric surgical resection, was obtained from the patients’ medical records, endoscopy reports, and pathology reports. Permission for the study was obtained from the institutional review board at our institution.
Pathologic features
We reviewed hematoxylin and eosin-stained slides prepared from routinely processed, formalin-fixed polypectomy specimens in all cases. The polyps were classified according to previously established criteria and evaluated for the presence of intestinal metaplasia and dysplasia. The latter was graded as low or high according to the WHO classification scheme and noted to be intestinal or foveolar type, as previously described.12 When available, concomitant and prior biopsy samples of the non-polypoid mucosa were evaluated for the presence of gastritis, H. pylori infection, and chemical gastropathy.
Immunohistochemical studies
Immunohistochemical stains were performed on 5 µm-thick formalin-fixed (10% buffered formalin), paraffin-embedded tissue sections using standard techniques and the antibodies enumerated in Table 1. Nuclear staining for MGMT, MLH1, MSH2, PMS2, MSH6, p27, p53, p16, and ß-catenin was evaluated in each of the polyps, and separately assessed in hyperplastic and dysplastic areas of polyps that contained both elements. A positive result for MGMT and mismatch repair proteins was defined as complete loss of staining in the lesional epithelium. Loss of staining for p27 in >10% of the lesional epithelium was considered a positive result, as reported by others.13 The extent of strong nuclear staining for p53 in the superficial epithelium was recorded and nuclear staining for ß-catenin was graded as absent (negative or weak staining) or present (moderate to strong staining). The extent of strong cytoplasmic and nuclear staining for p16 was also noted.
Immunohistochemical Stains |
||
Marker |
Dilution |
Company |
DNA Repair |
||
Complete Loss of MGMT |
1:25 |
NeoMarkers (Fremont, CA) |
Complete Loss of MLH-1 |
1:25 |
BD Biosciences (San Jose, CA) |
Complete Loss of MSH-2 |
0.18055556 |
Calbiochem (Cambridge, MA) |
Complete Loss of MSH-6 |
0.18055556 |
BD Biosciences (San Jose, CA) |
Cell Cycle Regulation |
||
Loss Of P27 |
1:25 |
Biocare Medical (Concord, CA) |
Increased P16 (Nucleus and Cytoplasm) |
Prediluted |
MTM Laboratories (Westborough, MA) |
Tumor Suppressor Function |
||
Nuclear P53 Staining |
1:200 |
BioGenex (San Ramon, CA) |
Wnt Signaling |
||
Nuclear b-Catenin Staining |
1:200 |
BD Biosciences (San Jose, CA) |
Primers Used for PCR Amplification and Sequencing |
||
Gene Evaluated |
Forward |
Reverse |
b-catenin (CTNNB1) Exon 3 |
ATGGAACCAGACAGAAAAGC |
GCTACTTGTTCTTGAGTGAAG |
APC Segment 1 |
CAGACTTATTGTGTAGAAGA |
CTCCTGAAGAAAATTCAACA |
APC Segment 2 |
AGGGTTCTAGTTTATCTTCA |
TCTGCTTGGTGGCATGGTTT |
APC Segment 3 |
TAAGTGGCATTATAAGCCCCAGTG |
TGTATAAATGGCTCATCGAGGCTC |
APC Segment 4 |
ACTCCAGATGGATTTTCTTG |
GGCTGGCTTTTTGCTTTAC |
KRAS (codons 12 and 13) |
CGTCTGCAGTCAACTGGAAT |
AGAATGGTCCTGCACCAGTAA |
BRAF (V600E) |
TGCTTGCTCTGATAGGAAAATG |
GACTTTCTAGTAACTCAGCAGC |
Table 1 Immunohistochemical reagents and PCR primers used in the evaluation of gastric polyps
Molecular analysis
Molecular studies were performed on adenomas and hyperplastic polyps spanning >1 cm. For each case, the non-lesional tissue was manually removed from unstained sections mounted on glass slides and DNA was extracted from the remaining tissue using the QIAamp DNA MiniKit (Qiagen Sciences, Valencia, CA), followed by PCR amplification. Hyperplastic and dysplastic epithelia were separately dissected from polyps with both types of elements. The primer sequences, all designed to amplify PCR amplicons <400 bp in size, are enumerated in Table 1. Polyps with dysplasia, and those that showed nuclear ß-catenin staining, were subjected to mutational analysis of exon 3 (glycogen synthetase kinase-3ß phosphorylation region) of CTNNB1 and the APC mutation cluster region (exon 15, codons 1260-1596). The latter was analyzed in overlapping PCR segments with four sets of paired primers. Extracted DNA was also analyzed for mutations in selected regions of KRAS (codons 12 and 13) and BRAF (V600E). PCR products were sequenced bi-directionally using the Big Dye Terminator chemistry and Applied Bio systems Automated 3730 DNA analyzer and analyzed with the aid of Mutation SurveyorTM software (Soft Genetics, State College, PA).
Statistical analysis
The clinical, immunohistochemical and molecular features of each group were tallied and compared using the Fisher’s exact test. A p value <0.05 was considered statistically significant.
Clinical and pathologic features
The clinical and pathologic features of the study cases and controls are summarized in Table 2. Most patients were adults (mean age: 63 years, median: 71, range: 16-91) and females were more commonly affected (male/female ratio: 7/10). Most polyps in the study and control groups occurred in the antrum. All 15 large hyperplastic polyps contained tortuous, cystically dilated glands lined by foveolar-type epithelium and inflamed lamina propria (Figure 1). Four displayed foci of intestinal metaplasia, which were limited to the polyp in three cases and present in background mucosa in one case. Four (27%) large polyps in the study group contained areas of low-grade dysplasia, one of which also showed high-grade dysplasia (Figure 1). Prior or concomitant biopsy samples of non-polypoid gastric mucosa were available for review in twelve study polyps (80%), including eight without dysplasia and all four with dysplasia. In eight study polyps without dysplasia, the background mucosa showed gastric injury in six (75%), including three (38%) with H. pylori-related chronic gastritis and three (38%) with chemical gastropathy. Of the four study polyps with dysplasia, the background mucosa showed gastric injury in two (50%), including one (25%) with chemical gastropathy. Eight patients with small hyperplastic polyps had prior, or concomitant, gastric mucosal biopsies of non-polypoid mucosa. Of these, three (38%) displayed chemical gastropathy, two (25%) contained intestinal metaplasia, and one (13%) showed H. pylori-negative chronic gastritis. All gastric adenomas displayed low-grade dysplasia. These patients had chronic gastritis (89%) and H. pylori infection (22%) in the non-polypoid mucosa at rates similar to those of the study group, although intestinal metaplasia was more frequent (67%).
Feature Evaluated |
Large Hyperplastic Polyps without Dysplasia |
Large Hyperplastic Polyps with Dysplasia |
Small Hyperplastic Polyps |
Adenomas |
Mean Age (years) |
66 |
62 |
59 |
64 |
Male/Female Ratio |
7-Apr |
2-Feb |
6-Apr |
5-Apr |
Polyp Location |
||||
Cardia |
3/11 (27%) |
0/4 (0%) |
1/10 (10%) |
0/9 (0%) |
Body/Fundus |
2/11 (18%) |
2/4 (50%) |
4/10 (40%) |
3/9(33%) |
Antrum |
6/11 (55%) |
2/4 (50%) |
5/10 (50%) |
6/9 (67%) |
Mean Size |
1.5 cm |
3.8 cm |
0.9 cm |
1.7 cm |
Chronic Gastritis |
5/7 (71%) |
2/4 (50%) |
1/8 (13%) |
8/9 (89%) |
H. pyloriInfection |
3/7 (43%) |
0/4 (0%) |
0/8 (0%) |
2/9 (22%) |
Autoimmune Gastritis |
0/7 (0%) |
0/4 (0%) |
0/8 (0%) |
1/9 (11%) |
Chemical Gastropathy |
3/7 (43%) |
1/4 (25%) |
3/8 (38%) |
2/9 (22%) |
Intestinal Metaplasia |
3/7 (43%) |
1/4 (25%) |
2/8 (25%) |
6/9 (67%) |
Table 2 Clinical and pathologic features of gastric hyperplastic and adenomatous polyps
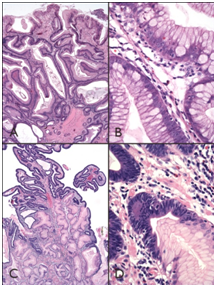
Figure 1
1A) Hyperplastic polyps contain tortuous, somewhat dilated glands enmeshed within edematous and inflamed lamina propria.
1B) Epithelial cells may show mucin depletion, or contain abundant cytoplasmic mucin, with mild cytologic atypia.
1C) Four large hyperplastic polyps contained foci of dysplasia.
1D) Which was generally low-grade and displayed nuclear enlargement, hyperchromasia, and increased mitotic activity.
Immunohistochemical and molecular features
DNA repair mechanisms: The immunohistochemical and molecular features of the study cases and controls are summarized in Table 3. Complete loss of MGMT staining was observed in seven (47%) large hyperplastic polyps, including two with foci of dysplasia that showed loss of MGMT staining in both non-dysplastic and dysplastic areas (Figure 2A & 2C). In contrast, all 10 small hyperplastic polyps and nine adenomas showed preserved MGMT staining (p=0.02 versus study polyps). Only one (11%) gastric adenoma showed complete loss of MLH-1 expression, but all remaining study cases and controls displayed preserved staining for MLH1.
Large Hyperplastic Polyps without Dysplasia |
Large Hyperplastic Polyps with Dysplasia |
Small Hyperplastic Polyps |
Adenomas |
||
Hyperplastic Areas |
Dysplastic Areas |
||||
DNA Repair Mechanisms |
|||||
Loss of MGMT |
5/11 (45%) |
2/4 (50%) |
2/4 (50%) |
0 (0%)* |
0 (0%)* |
Loss of MLH-1 |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
1/9 (11%) |
Loss of MSH-2 |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
Loss of MSH-6 |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
Cell Cycle Regulators |
|||||
Loss of p27 |
5/11 (45%) |
2/4 (50%) |
2/4 (50%) |
1/10 (10%) |
0 (0%)* |
Increased p16 |
5/11 (45%) |
0/4 (0%) |
1/4 (25%) |
0 (0%) |
3/9 (33%) |
Tumor Suppressor Function |
|||||
Increased p53 (>10%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
Wnt Signaling Pathway |
|||||
Immunohistochemistry |
|||||
Nuclear b-catenin |
0 (0%) |
0 (0%) |
2/4 (50%) |
0 (0%) |
4/9 (44%) |
Molecular analysis§ |
N=3 |
N=3 |
N=5 |
||
APC Mutation |
N/A |
1 case |
2 cases |
N/A |
5 cases |
b-catenin Mutation |
N/A |
1 case |
1 case |
N/A |
0 cases |
MAP Kinase Pathway |
|||||
KRAS Mutation |
1/11 (9%) |
0 (0%) |
1/4 (25%) |
N/A |
0 (0%) |
BRAF Mutation |
1/11 (9%) |
0 (0%) |
0 (0%) |
N/A |
1/9 (11%) |
Table 3 Immunohistochemical and molecular features of hyperplastic and dysplastic polyps
*p=0.02, study polyps versus small hyperplastic polyps and adenomas
§Discrepancies in total numbers are due to failed analyses and/or insufficient material
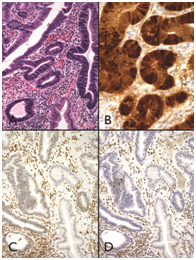
Figure 2
1A) One large hyperplastic polyp with dysplasia.
1B) Showed nuclear b-catenin staining that reflected an underlying b-catenin mutation.
1C) This lesion also showed concomitant loss of MGMT.
1D) p27 immunohistochemical staining in both non-dysplastic and dysplastic epithelium.
Cell cycle regulation: Seven (47%) study polyps showed loss of p27 staining, including two with dysplasia that showed decreased expression in both hyperplastic and dysplastic elements (Figure 2D). Five (71%) study cases with loss of p27 staining showed concomitant loss of MGMT staining. In contrast, p27 staining was preserved in most (90%) small hyperplastic polyps and all adenomas (p=0.09and p=0.02 versus study polyps, respectively). Nuclear and cytoplasmic p16 staining was observed in 40% of study polyps, including one with dysplasia and one-third of adenomas, but none (0%) of the small hyperplastic polyps (Figure 3A & 3B). When present, p16 staining was detected in <10% of the polyp epithelium and frequently corresponded to moderate or strong nuclear staining for p53 (Figure 3C & 3D). None of the study cases or controls showed p53 staining in >10% of the lesional epithelium.
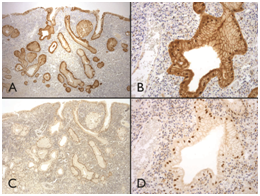
Figure 3
1A & 1B) Immunohistochemical studies demonstrated patchy strong nuclear and cytoplasmic p16 staining.
1C &1D) Which frequently corresponded to moderate or strong nuclear staining for p53 in virtually every cell.
Wnt signaling pathway: All large hyperplastic polyps without dysplasia showed membranous ß-catenin staining, whereas nuclear ß-catenin staining was seen in dysplastic areas of two study cases, which also showed mutations in APC (one case) and ß-catenin (one case) in dysplastic and non-dysplastic areas as well as concomitant loss of MGMT staining (Figure 2B). Another large hyperplastic polyp harbored an APC mutation limited to areas of dysplasia although nuclear ß-catenin staining was not seen in this case. Five of nine gastric adenomas were successfully analyzed for APC mutations and all five harbored truncating mutations; ß-catenin mutations were not detected in any of these polyps.
Mitogen Activated Protein (MAP) kinase pathway: Two study cases showed KRAS mutations, including one with a mutation confined to a dysplastic area, and one large hyperplastic polyp contained a BRAF mutation. Only one (11%) gastric adenoma contained a BRAF mutation; all of the rest showed no abnormalities in BRAF or KRAS.
We evaluated the clinical, immuno phenotypic and molecular features of large (>1 cm) gastric hyperplastic polyps and compared them to the features of small (=1 cm) hyperplastic polyps and gastric adenomas. We found that large hyperplastic polyps are more frequently associated with chronic gastritis compared to small polyps (58% versus 13%, p=0.07) and show some distinct molecular features. Large hyperplastic polyps commonly display loss of MGMT staining (47% vs. 0% compared to small polyps, p=0.02), often in combination with loss of p27 (47% vs. 10% compared to small polyps p=0.09), whereas these features were uncommon among gastric adenomas [0% and 0%, (p=0.02) for both comparisons]. Large hyperplastic polyps with dysplasia also display abnormalities in the Wnt signaling pathway, as evidenced by either ß-catenin or APC mutations. At variance with hyperplastic polyps of the colon, KRAS and BRAF mutations are infrequent among hyperplastic and dysplastic polyps of the stomach, suggesting that these mutations are of limited importance in their pathogenesis.
Gastric hyperplastic polyps have been incompletely studied, particularly in comparison to “hyperplastic” polyps of the colorectum. They are generally considered to represent non-neoplastic reparative-type lesions resulting from mucosal inflammation. In a study of 45 patients with gastric hyperplastic polyps, Borch et al.14 found 91% to have underlying chronic gastritis, particularly H. pylori-related gastritis (69%).14 Dirschmid et al.15 evaluated the background mucosa of 244 patients with gastric hyperplastic polyps and found 51% and 37% to have autoimmune and H. pylori-associated gastritis, respectively.15 Finally, Abraham et al.4 reported that 85% of hyperplastic polyps developed in combination with chronic gastritis and 37% occurred in mucosae with intestinal metaplasia.4 Our results are similar, although we found small hyperplastic polyps to be more common in cases of chemical gastropathy, whereas large hyperplastic polyps were more frequently associated with chronic gastritis.
Gastric hyperplastic polyps occasionally contain foci of dysplasia. Some describe dysplasia in nearly 20% of cases, although the true prevalence of dysplasia in these lesions is likely less than 5%.4,16-18 Data from several studies including our own suggest that dysplasia is more common in larger hyperplastic polyps.19,20 The mechanisms by which dysplasia develops in gastric hyperplastic polyps may be similar to other epithelial neoplasms of the gastrointestinal tract. Hypermethylated promoter regions of mismatch repair genes such as MLH1 and MGMT have been reported in up to 10% of gastric adenomas, although MSI-H is less frequently identified in 3% of cases.21-23 Only one study to date has examined gastric hyperplastic polyps for mismatch repair deficiency. Nogueira et al.23 evaluated six hyperplastic polyps for MSI and found only one case with instability at one locus (BAT-26).24 We failed to demonstrate evidence for functional mismatch repair deficiency in hyperplastic polyps of our study. None of the gastric hyperplastic polyps we examined showed loss of MLH-1 immunostain, although nearly 50% of large polyps showed complete loss of MGMT expression by immunohistochemistry.
Cyclin dependent kinase inhibitor 1B (CDKN1B, p27) prevents progression into S-phase of the cell cycle and may be important in progression of gastrointestinal neoplasia, particularly in cancers that show extensive DNA hypermethylation.25 Gastric adenomas and carcinomas commonly display loss of p27 immunohistochemical staining (up to 30% and 50%, respectively).26,27 In our study, 47% of large hyperplastic polyps but only 10% of small hyperplastic polyps, showed loss of p27 staining. Most of these cases (71%), including one hyperplastic polyp with dysplasia, showed concomitant loss of MGMT staining. Although not conclusive, these findings may point to DNA methylation in the development of large hyperplastic polyps of the stomach.
Abnormalities in the MAP kinase pathway play a minor role in gastric carcinogenesis; less than 5% of gastric cancers harbor either KRAS or BRAF mutations.28 The prevalence of KRAS mutations among gastric adenomas is also low, whereas data regarding mutations among hyperplastic polyps are variable.22,29 Murakami et al.19 found only one KRAS mutation among 17 hyperplastic polyps with dysplasia, but Dijkhuizen et al.29 found identical KRAS mutations in three concurrent hyperplastic polyps with high-grade dysplasia from one patient.19,30 We found MAP kinase pathway abnormalities in 20% of large hyperplastic polyps, including two polyps without dysplasia (one having a BRAF mutation and one having a KRAS mutation), and one polyp with dysplasia and a KRAS mutation.
Emerging data suggest that alterations in the Wnt signaling pathway, particularly mutational inactivation of APC, are important to the evolution of gastric epithelial neoplasia.22,29,31 Lee et al.22 evaluated 78 cases of gastric dysplasia and found APC mutations in 76% of cases, but did not detect CTNNB1 mutations in any of the lesions.22 Similarly, we detected Wnt signaling abnormalities in all gastric adenomas and 67% of hyperplastic polyps with dysplasia that were tested: two had APC mutations and one had CTNNB1 mutation. Experience with colorectal neoplasia suggests that alterations in ß-catenin are infrequent compared to APC mutations, but may be more common among subsets of colon cancer with MSI. Further studies exploring the relationships between MGMT and p27 expression, ß-cateninmutation, DNA methylation, and MSI-H in gastric cancers may elucidate the roles of these factors in gastric carcinogenesis.
Based on our findings and published information, it appears that large gastric hyperplastic polyps are not merely larger versions of small hyperplastic polyps, but biologically different lesions. Features favoring this interpretation include the not infrequent finding of dysplasia in large polyps, their association with inflammation and intestinal metaplasia, and the presence of immuno phenotypic and molecular abnormalities that are more commonly found in neoplastic conditions. Of interest, a study using comparative genomic hybridization showed a similar frequency of chromosomal instability in gastric adenomas and hyperplastic polyps with intraepithelial neoplasia suggesting the possible existence of at least two mechanisms of gastric carcinogenesis, similar to the chromosomal instability and serrated pathways of colorectal carcinogenesis.32
In summary, our results suggest that small gastric hyperplastic polyps are probably non-neoplastic lesions. However, large hyperplastic polyps frequently develop in association with chronic gastritis, show molecular abnormalities, and may even contain foci of dysplasia. Most commonly, large hyperplastic polyps display concomitant loss of MGMT and p27 expression. Abnormalities in Wnt signaling, including nuclear ß-catenin immuno expression and/or APC or ß-catenin mutations, are often detectable in both hyperplastic and dysplastic epithelia of polyps that contain mixed elements. These findings suggest that a subset of large hyperplastic polyps may represent neoplastic lesions that develop secondary to long-standing mucosal inflammatory injury.
None.
The author declares no conflict of interest.

©2017 Saab, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.