Advances in
eISSN: 2573-2862


Short Communication Volume 3 Issue 1
Cellular Pathology, Oxford University Hospitals NHS Foundation Trust, UK
Correspondence: Katharine Sheppard, Cellular Pathology, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Headley Way, Headington, Oxford, OX3 9DU, UK, Tel 01865 220581
Received: November 14, 2017 | Published: January 10, 2018
Citation: Sheppard K, Bond S, Manek S. Diagnostic challenge of cervical intraepithelial neoplasia with concurrent follicular cervicitis: histomorphological features and p16 immunostaining as a diagnostic adjunct. Adv Cytol Pathol. 2018;3(1):1-3. DOI: 10.15406/acp.2018.03.00041
There are diagnostic challenges in identifying cervical intraepithelial neoplasia (CIN) in squamous epithelium in the presence of dense cervical stromal inflammation. We identified common histomorphological features in concomitant cervical intraepithelial neoplasia and follicular cervicitis, using p16INK4a immunohistochemistry to guide accurate diagnosis of CIN Eighteen diathermy loop excision specimens were examined, and representative blocks with follicular cervicitis stained with p16INK4a immunohistochemistry. We found difficulties in accurately diagnosing CIN in the epithelium overlying areas of follicular cervicitis. Two common patterns of histomorphological features were identified and termed ‘Thin CIN’ and ‘Spongiotic CIN’. p16 was found to be a valuable diagnostic adjunct in this context.
Keywords: Uterine cervicitis; Cervical intraepithelial neoplasia; p16; Pathology
CIN: Cervical Intraepithelial Neoplasia; FC: Follicular Cervicitis
The literature acknowledges difficulties in diagnosing cervical intraepithelial neoplasia (CIN) when it is closely associated with a dense chronic inflammatory infiltrate within the superficial cervical stroma. This is due to atypical reactive and regenerative epithelial changes such as nuclear enlargement/overlap and impaired maturation, mimicking some of the cytological and architectural features found in CIN. 1,2 Follicular cervicitis (FC), a form of chronic cervicitis with prominent lymphoid follicles containing well-formed germinal centres, is usually easily identifiable but has led to misdiagnoses in gynecologic cytology,3 and we propose also in cervical histology. p16INK4a tumour-suppressor protein over expression has been shown to detect integrated high-risk human papilloma virus (hrHPV), with p16INK4a immunohistochemistry (p16 IHC) acting as a surrogate biomarker for oncogenic hrHPV infection.4,5 In the supporting literature and the authors’ experience, p16 immunoreactivity tends to be weak and patchy in low grade CIN (CIN1) and reactive mimickers, such as atrophy and squamous metaplasia. Conversely strong and diffuse (so-called “block-positive” staining) p16 immunoreactivity strongly favours an interpretation of high grade CIN (CIN2 and CIN3).1-7 Consensus recommendations from the Lower Anogenital Squamous Terminology (LAST) project from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology advise pathologists to consider using p16 IHC for equivocal lesions only; when the H&E morphologic differential diagnosis is between a high grade disease and either a high grade mimic, such as immature squamous metaplasia or low grade disease. They state that although the grade of CIN should be based on the H&E histomorphology of the lesion, if a biomarker such as p16 IHC is used, the results may override the original H&E interpretation. Routine use of p16 IHC is not recommended, especially when the H&E morphologic differential diagnosis is between low grade disease and negative. Overuse of p16 IHC might lead to the potential overtreatment of patients following overinterpretation of staining patterns in low grade lesions.8 The aim of this study was to identify common histomorphological features in coexisting CIN and FC, which to our best knowledge have not been previously demonstrated.
The electronic patient record at Oxford University Hospitals NHS Foundation Trust was used to retrieve all diathermy loop excision (DLE) specimens with concomitant CIN and FC over a two-year period. All original haematoxylin and eosin (H&E)-stained slides were examined from the DLEs. Only cases of FC showing secondary lymphoid follicles with well-formed germinal centres within the superficial stroma as part of the chronic inflammatory infiltrate and background classical high grade CIN (CIN 2 and CIN 3) were included. Twenty DLEs showed both diagnoses and two cases were excluded. p16 IHC was performed on one representative block of FC from each DLE. The pattern of p16 immunostaining and histological features in the squamous epithelium associated with the FC was recorded.
In only 4 out of the 18 specimens, CIN appeared unaffected by FC. In 9 excision specimens’ epithelial spongiosis, thinning and ulceration were identified concomitantly within the epithelium overlying and adjacent to the secondary lymphoid follicles of FC. Twelve excisions with FC-associated epithelial spongiosis +/- intraepithelial inflammatory cells (Figure 1a & 1b), and 13 excisions with FC-associated epithelial thinning (epithelium < 10 cells thick) +/- ulceration (Figure 2a & 2b) were strongly and diffusely positive for p16 IHC in areas where epithelial atypia was not readily apparent on the H&E sections. In 1 case, spongiosis obscured the extent of dysplasia, and p16 immunostaining lead to a revision of this area from CIN1 to CIN3. In another, histomorphological features suspicious of high grade CIN within thin epithelium on the H&E section were seen, but the area was negative for strong diffuse p16 IHC, and the epithelial atypia interpreted as a reactive (Figure 3a & 3b). As expected, in the majority of cases there were difficulties in accurately diagnosing CIN in the epithelium overlying areas of FC. This was particularly true in the presence of two distinct histological patterns often found together overlying areas of FC and dense chronic inflammation within the cervical stroma: epithelial thinning and epithelial spongiosis. We propose the terms ‘Spongiotic CIN’ and ‘Thin CIN’ to describe covert CIN within reactive and reparative-appearing cervical squamous epithelium that can be seen in inflammatory processes such as follicular cervicitis. We believe that if p16 IHC is used judiciously, when either high grade CIN is suspected but not unequivocal on H&E sections, or when assessment of the epithelium is compromised by intense cervical stromal inflammation such as in follicular cervicitis, p16 IHC is a valuable diagnostic adjunct. The role of inflammation and host’s immune response in cancer has been increasingly acknowledged. However, the interplay between HPV infection, CIN, the local mucosal and systemic immune response, immune dysregulation and the local cervical-vaginal milieu is complex and our understanding is still incomplete. Cervical inflammation plays a part in the pathogenesis of CIN 9-11 and has been cited as a co-factor in cervical carcinogenesis. 9 Increasing levels of inflammation and advancing hrHPV-related CIN grade are correlated.9,10,12 But confusingly, various host inflammatory responses are implicated in the clearance and persistence of HPV infection, and the progression and regression of hrHPV-related CIN!. 9-12 Further investigation is needed into the association between cervical inflammation and dysplasia.
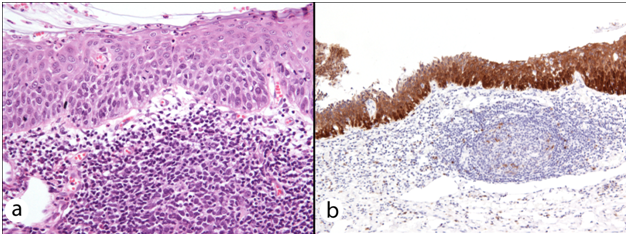
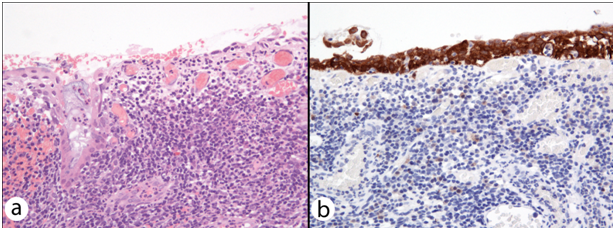

This has been the first study to demonstrate two common histomorphological patterns of CIN overlying and adjacent to FC. Recognition of ‘Thin CIN’ and ‘Spongiotic CIN’ patterns has important diagnostic implications. These patterns may seem incidental when classical CIN is also found in the H&E sections. However, CIN with FC may be erroneously graded (as in one of our cases), overlooked at margins or even disregarded entirely when classical CIN is not present. Therefore, the presence of dense inflammation with secondary lymphoid follicle formation in any cervical specimen lined by squamous epithelium should raise the question of ‘Thin CIN’ or ‘Spongiotic CIN’, and prompt consideration of the use of p16 immunostaining to guide accurate diagnosis of dysplasia.
None
The authors declare no conflicts of interest and no sources of funding.

©2018 Sheppard, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.