Advances in
eISSN: 2573-2862


Mini Review Volume 3 Issue 4
Institute of Food Biotechnology and Genomics, Ukraine
Correspondence: Kravets EA, Institute of Food Biotechnology and Genomics, National Academy of Sciences of Ukraine, 2A Osypovskyi Str, Kyiv 04123, Ukraine, Tel 044 434 3777
Received: March 13, 2018 | Published: September 5, 2018
Citation: Kravets EA. Cytomixis as a primary form of sexual process. Adv Cytol Pathol. 2018;3(4):88-91 DOI: 10.15406/acp.2018.03.00059
Background: In this review presents a new concept of the functional role of cytomixis. Anther is a self-organizing system where microsporocytes demonstrate certain traits of individuality and social behaviour, in particular altruism, competition and collective self-organization. Cytomixis function in microsporogenesis is comparable with evolutionarily primary and primitive form of sexual process where genetic recombination, DNA repair, cell-to-cell transfer and mixing of genes are happen creating a mixed gene pool of a microsporocytes population. Owing to cytomixis, microsporocytes not only get rid of excess mutations, maintain their polymorphism and heterozygosity, but also form an evolutional reserve.
Keywords: cytomixis, microsporocytes, DNA repair, gene transfer, mixing gene pool
Cytomixis is known as a type of cell-to-cell interaction through the exchange of nuclear and cytoplasmic material, typical of both plant and animal tissues. The most numerous studies regarding cytomixis are researches on microsporogenesis in angiosperms where it is described in more than 400 species from 84 families.1,2 The fact of cytomixis being attributed to microsporogenesis, especially to its early prophase is noticeable, indeed. It is known that it is in the prophase where meiosis key events occur: the approaching of homologues, their pair wise conjugation and crossing over. These cascading events are initiated by double-strand DNA breaks, including those induced by meiosis program.3
Cytomixis, while accompanying early-prophase chromosome transformations and being a part of them at the same time, must simultaneously correspond to their kinetics and be a subject to genetic regulation of meiosis. However, not everything is clear in the genetic regulation of cytomixis. The genes involved in the regulation of homologous segregation and chromosome organization in meiosis can be involved in genetic control of cytomixis,4,5 for example, DIF1 in Arabidopsis thaliana.6 However, there is no direct evidence that Mei gene mutations cause cytomictic migration of chromatin in prophase of meiosis.
If cytomixis is subject to the genetic regulation of meiosis, then migratory chromatin, given its intactness, can be integrated into a genome and functional unit of the recipient cells and participate in the reconstruction of microsporocytes (MMC) genome. This can be confirmed by chromosomal polymorphism of microsporocytes and pollen grains as well as by existence of a large variety of natural polyploid cytotypes in many plant species of which cytomixis is typical.7?12
The issue of cytomixis function and its effect on the course of meiosis also is not completely clear too. Does cytomixis stabilize or destabilize meiosis? Does it have a negative effect on pollen grains formation or contribute to optimization of their number and quality, shaping and adaptation through selection? It should be taken into account that the relationship between cytomixis and viability of pollen varies from species to species. It can be negative, positive or non-existent at all.8,13?16
On transgenic tobacco lines showed that cytomictic chromatin retains not only its physical, but functional properties as well.17-19 The chromosomal polymorphism of microsporocytes and pollen grains, the existence of a large variety of natural polyploid cytotypes in many cytomictic species as well as fact that cytomixis is attributed to male generative sphere and “male-driven” evolution allow us to consider cytomixis as one of the mechanisms to increase genetic diversity and speciation in plants.2,8-12,16-18,20?22 The wide occurrence of cytomixis in terrestrial plants testifies its being involved in the important genomic reorganization processes, which allow its carriers to be maintained due to natural selection process. However, despite significant progress in the investigation of cytomixis, its functional role is still not entirely clear.
Cytomixis is known as a form of cell-to-cell nuclear migration that involves the interaction of dynamic cytoskeletal components with the nucleus through signalling systems and linker complexes.23,24 However the role of cytoskeletal components in the cytomixis was poorly studied. Regarding functioning and cytological picture in monocots (e.g. lily, onion, barley), we differentiated cytomixis according to three types of interactions, namely pair-looped, donor-recipient and chained type.25,26 These varieties may occur within a single population of microsporocytes either sequentially or simultaneously. Apparently, pair-looped and donor-recipient interactions are more typical of spontaneous cytomixis, whereas chained interactions of induced one.
Pair-looped interactions can be observed in pre-meiotic interphase, early leptotene and zygotene and are often accompanied by microsporocyte polarization (Figure 1A-C) and partial “uncoiling” of coiled chromosomes. In the process of microsporocyte polarization we observed a sharp jogging of the nucleus to the cell wall. The loops contacting nuclei and chromosomes keep their normal structure corresponding to zygotene organization (Figure 1C). Micronuclei appear simultaneously with increased cytomixis activity in pair-looped interactions. As a rule, after completion of pachytene-zygotene polarized nuclei go back to their previous positions in adjacent cells, where they continue meiotic division. In some cases, the paired contacts are accompanied by conjunction of the nuclei and even merging nucleoli. Structurally, cytomixis is a part of the early-prophase chromosomes transformations, whereas functionally, we suppose, of DNA monitoring, homologous (perhaps, as well other ones types) recombination and DNA repair.
In the donor-recipient variety, cytomixis resembles a primitive form of sexual reproduction of prokaryote conjugation (when the donor is active) or transformation (when the recipient is active). Indeed, in the first case, the donor (one or more) injects its chromatin into another microsporocyte, with the recipient remaining passive (Figure 1D)(Figure 1E). Typically, chromatin of the nuclei and micronuclei gets compacted in both donor and recipient. In the second case, in the transformation process, the recipient often keeps its nucleus structure in accordance with the stage of meiotic prophase, while the donor’s chromatin is getting compacted (Figure 1F). Condensation and agglutination of chromatin are often reversible transformations. Perhaps the donor-recipient cytomixis is a continuation of pair- looped interactions in the case DNA repair has not been completed. The donor DNA probably not only serves a matrix for homologous recombination and repair of DS DNA breaks, but can be integrated into the host genome, introducing additional genes similarly to that occurring in horizontal gene transfer. At the same time, gene transfer can be fragmented or genome-wide, unilateral, one-sided, double-sided or chained. In the donor-recipient relationships, collective altruistic behaviour of microsporocytes can be discerned, as well as traits of collective self-organization in chained interactions.
Chained interactions involve several or sometimes significant number of cells (Figure 1G)(Figure 1H). Inter-cellular contacts are accompanied by the appearance of micronuclei, which are formed from migrating chromatin across cytomictic canals. Cell-to-cell interaction involves all the nucleus components including nucleoli (Figure 1H) that suggest preservation of native and functional activity of cytomictic chromatin and its probable contribution to the protein synthesis in recipient cells. Migratory nuclei become involved in the chained migration while crossing more than one cell on their way. The volume of decreasing chromatin in such interactions is probably compensated with the income one. It is obvious that at least a part of hypo- and hyper-chromosome microsporocytes can restore their genetic balance. It is logical to assume that it is in chained interactions where “shuffling” of genes and intra-tissue “mixing” of microsporocyte genomes occur, which can involve the DNA fragments belonging to different cells. New gene combinations increase heterozygosity and renew gene pool of a microsporocytes population. It is obvious that “gene mixing” supports genetic homeostasis of microsporocytes.
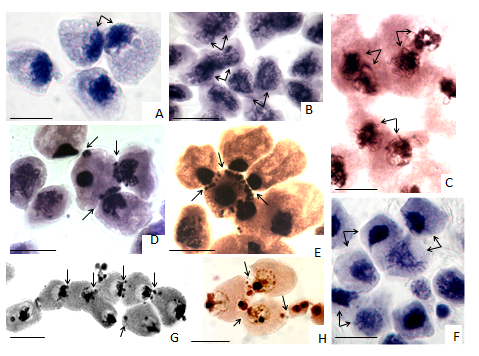
Figure 1 Structural-functional types of cytomixis of microsporocytes in zygotene: (A-C) polarization and pair-looped interactions (arrows), (D-F) donor-recipient microsporocyte interactions in zygotene-pachytene (arrows), (G-H) chained interactions of microsporocytes, involvement of nucleoli in nuclear translocation (h, arrows); A. fistulosum (A, B), A. cepa (F), H. distichum (C, G, H), L. croceum (D, E). Transmission light acetohaematoxyline (A, B, D, E, I, G), acetocarmine (C) and silver nitrate staining (H). Bars represent 10 µm.
Multi-cell chained interactions are not rare in cytomictic migration. Thus, about 80% zygotene nuclei of tetraploid cytotype Lippia alba undergo cytomictic exchange.1 Formation of microsporocytes clusters containing from two to several tens of hypo- and hyper-chromosome (sometimes a nuclear) cells reflects the direction and extent of cytomictic migration in an anther.16 It is believed that cytomixis generates high-level heterozygosity in a male generative system.27,9 Also cytomixis supposedly contributes to the adjustments of a genome, adaptation and stabilization of new polyploids (so-called neuploids) due to large scale losses and gains of DNA.12
Thus pair-looped structural type of cytomixis, we believe, is related to monitoring, homologous recombination and DNA repair. Donor-recipient type is associated with horizontal gene transfer, replacement of non-repaired DNA fragments and insertion of new genes. And chained type is connected with mixing genes along with renovation of microsporocytes gene pool. Consequently, microsporocytes restore their genetic and tissue homeostasis through cytomixis. It is known that genetic homeostasis (Lerner homeostasis) is responsible for the maintenance of heterozygosity, polymorphism, and certain pace and direction of mutagenesis,28 whereas tissue homeostasis is responsible for the maintenance of cell number and tissue size as well as its healthy condition.29,30 With respect to a cell population within an organism, cytomixis can be regarded as a mechanism, through which the tissue gets rid of excess mutations, in this way supporting polymorphism, heterozygosity and creates a “genetic continuum” within tissue. Anther is a self-organizing system in which microsporocytes reveal traits of individualization (personality) capable of searching, collective behaviour and self-organization. Individualization of microsporocytes is accompanied by the deposition of callose in their wall, which is a molecular filter and change of their development program (diploid/haploid). Limited spatial and nutrient resources in anther are the limiting factors contributing to unity of microsporocyte cooperation and at the same time to aggravation of competition in this fashion directing the development of the system.
It is common knowledge that cytomixis is sporadically found in many tissues and organs, but more often in generative male tissue (sporogenous tissue and microsporocytes), epidermis, the tissues to be utilized (anther wall, endosperm, senescent or damaged leaves) and less often in proliferative tissues (apical meristems, pre-embryo) as well as in vitro tissue and cell culture.16,31?33 Apparently, a common peculiarity of the listed types of tissue is relatively high frequency of cell prolipheration and mutagenesis occurrence.
Among various reasons of cytomixis (at the cellular level) attention shall be drawn to high-haploid DNA content per nucleus in many cytomictic species (herbaceous monocots, especially Liliaceae). Importantly, increasing DNA content goes along with increasing its degree of damage and cell life cycle34 that may be associated with time spent for DNA repair and passing checkpoints. Furthermore, polyploidy and aneuploidy, as is has been known, may be not only the result but a cause of cytomixis.8,9,12,35 Finally, cytomixis in many cases is an induced process with environmental stimulus being a trigger.6,8,15,16,20,26 Cytomixis activization is promoted by system factors; such as weakening of cooperation, inter-level integration and regulatory links within an organism. To sum up, increasing mutagenesis rate and weakening of cell cooperation lead to tissue and genetic homeostasis disruption. Besides, it is hazardous for integration unity of an organism. In such circumstances cytomixis represents a radical mechanism of restoration of weakened genetic and tissue homeostasis in plant organs.
Synchronism in early-prophase chromosomes transformations dynamics and cytomictic migration indicates cytomixis participation in key events of prophase. Cytomixis is probably initiated by a cascade of signals that trigger the prophase reorganization of nucleus and cytoskeleton.23,24 However, gene transfer from one cell into another cannot be a random event, especially in the case of male gamete initial cells.
Cytomixis functions may include inter-cellular transfer of matrix for homologous repair of DS (double-strand) DNA breaks, gene transfer, which can be integrated into the recipient genome replacing unrepairable DNA fragments, shuffling genomes resulting in renewal of microsporocytes gene pool (Figure 2). While keeping polymorphism and heterozygosity microsporocytes population gets rid of genetic load. Hyper- and hypochromosome microsporocytes, unreduced and aneuploid PGs probably serve a material for selection (both negative and positive), making up a strategic reserve for possible evolutionary events. Abnormalities of meiosis resulted from cytomixis are kind of “draft” of the events. Extrusions and diminution of chromatin, syncytium are probably used as precursors for development of pollen grains.
Anther is a self-organizing system in which microsporocytes reveal traits of personality capable of complex collective behaviour and self-organization. Cytomixis can act as a primitive and evolutionarily primary sexual process in which DNA recombination and repair take place, harmful mutations are eliminated, gene transfer and mix occur creating a mixed gene pool, and in this fashion genetic and tissue homeostasis of microsporocytes is maintained. New genetic variants are first checked through haplontic, then through individual selection. The last picks up and eventually fixes adaptive genetic variants.
The work was partially supported by the Ukrainian Foundation for Basic Research (grant no. 46-04-14).
None.
The author declares that they have no competing interests.

©2018 Kravets. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.