Advances in
eISSN: 2573-2862


Research Article Volume 3 Issue 4
1Pathology Department, Zagazig University, Egypt
2Medical Oncology Department, Zagazig University, Egypt
3General Surgery Department, Zagazig University, Egypt
Correspondence: Mouhamed A Fouad, Department of Pathology, faculty of medicine, Zagazig university, Egypt, 44519, Tel 0102 3096 3123
Received: July 30, 2018 | Published: August 31, 2018
Citation: Fouad MA, Elfeky MA, Ahmed RZ, et al. Prognostic significance of ubiquitin-specific protease 22 (USP22), and stress-induced phosphor-protein-1 (STIP-1) expressions in papillary thyroid carcinoma; an immunohistochemical study. Adv Cytol Pathol. 2018;3(4):76-86 DOI: 10.15406/acp.2018.03.00058
Background: Papillary carcinoma of the thyroid gland (PTC) is a malignant thyroid neoplasm which had a favorable prognosis; but as it is a highly invasive cancer which has a capability to invade the surrounding tissues and spread to lymph-nodes, which leads to higher incidence of recurrence of the tumor and hematogenous spread which lead to dismal patient outcome. Discovering novel prognostic bio-markers for PTC can help to detect recurrence, invasion, predicting patient outcome and the possible use as therapeutic-targets for such type of cancer. Ubiquitin-specific protease 22 (USP22), is deubiquitinating enzyme which is associated with occurrence of metastasis and resistance to therapy. Stress-induced phosphor-protein-1 (STIP-1) that is also named heat-shock-protein-(HSP, cold interact with heat shock proteins to form structures which have roles in many biological processes. Both USP22 and STIP-1 are incriminated in progression of the cell cycle.
The purpose of the current study was to detect the expression levels of both USP22 and STIP-1 in samples of papillary thyroid carcinoma (PTC), to correlate their combined expression with pathological and follow-up parameters to evaluate its clinical significance in PTC patients.
Methods: We evaluated USP22 and STIP-1 expression levels in sections from 30 paraffin blocks of PTC using immunohistochemistry, analyzed correlations between their expression levels, clinic-pathological and follow-up parameters of patients with PTC.
Results: Increased USP22 expression was associated with larger PTC size (p=0.02 and 0.03 respectively), presence of extra-capsular invasion (p=0.004), multi-focality (p=0.002 and 0.003 respectively), extrathyroidal extension (p=0.006 and 0.009 respectively), presence of lymph node metastasis (p=0.03 and 0.034 respectively), presence of vascular invasion (p=0.008 and 0.002 respectively), presence of distant metastasis (p=0.042 and 0.006 respectively) ans advanced stage (p=0.012 and 0.018 respectively).
USP22 expression was associated with higher incidence of loco-regional recurrence (LRR), neck node recurrence (p=0.26 and 0.003 respectively).
PTC patients with high USP22 expression had a shorter OS & DFS when compared to patients with low USP22 expression (p=0.014 and 0.006 respectively).
We have found that PTC patients with high STIP-1 expression had a shorter OS & DFS when compared to patients with low STIP-1 expression (p=0.014 and 0.043 respectively).
Expressions of both USP22 and STIP-1 were significantly positively correlated with each other (p=0.01).
Conclusion: Tissue protein expressions of both USP22 and STIP-1 were independent poor prognostic factors for PTC patients.
Keywords: papillary-thyroid-carcinoma, USP22, STIP-1, prognosis, immunohistochemistry
Malignant thyroid neoplasm is the commonest cancer of the endocrine system forming 3% of all human malignancies. Its incidence is markedly increasing in several countries in the past decades.1 The commonest subtype of malignant thyroid neoplasm is the papillary thyroid carcinoma (PTC) which forms about 80–85% of well-differentiated thyroid malignancies. Most PTC patients have a favorable outcome but there is a high incidence of early invasion of the nearby structure in certain cases.2 So it will be beneficial to detect novel biomarkers which could detect cases of PTC with higher incidence of invasion and metastasis. The novel ubiquitin hydrolase, which is named ubiquitin specific protease 22 (USP22), is a subunit of the human Spt-Ada-Gcn5-acetyltransferase activator complex and it is a component of a cancer stem cell signature.3,4 USP22 has been demonstrated to remove ubiquitin efficiently from the core histone H2A and H2B and could regulate transcription by alterations in histone ubiquitylation levels.5,6 It was found that knocking down of USP22 decreased cell cycle progression and strengthens the liability cancerous growth, invasion and metastases.4,6 So, USP22 might be incriminated in oncogenesis, increases tumor progression, and it becomes a novel attractive therapeutic target for cancers of many organs. But its role in cell cycle progression and progression of PTC has not been clarified yet. Stress-induced phosphor-protein-1 (STIP-1) that is also named heat-shock-protein-(HSP, cold interact with heat shock proteins to form structures which have roles in many biological processes e.g.; cell cycles progression, signal transductions, transcriptions, protein folding and RNA splicing.7‒9 STIP-1 tissue protein expression has been evaluated in plethora of cancers,10 those points to its role in carcinogenic process and the possibility of considering it novel therapeutic targets for cancer.11 There are few studies which assessed its clico-pathological and prognostic role in PTC.
The purpose of the current study was to detect the expression levels of both USP22 and STIP-1 in samples of papillary thyroid carcinoma (PTC), to correlate their combined expression with pathological and follow-up parameters to evaluate its clinical significance in PTC patients.
The current study was a prospective cohort study where; we included 30 samples of PTC which were retrieved from 30 patients that were surgically managed in department of General Surgery, Faculty of Medicine, Zagazig University. Specimens were sent to Pathology Department Faculty of Medicine, Zagazig. Routine hematoxylin & Eosin staining was done then immune-histochemical staining and evaluation of USP22 and STIP-1 expressions were done on 30 paraffin blocks of all the 30 patients with PTC. We have followed the 30 patients with PTC for 5 years from March 2013 to March 2018 for assessment of response to therapy, recurrence, progression of the disease and detection of survival rates to correlate all these parameters with the studied immunohistochemical markers in both departments of Medical Oncology, Faculty of Medicine, Zagazig University. We used TNM staging-system modified by the eighth edition AJCC Cancer Staging for surgical staging of PTC.12
The technique of streptavidine-biotin was used,13 where USP22 and STIP-1 tissue protein expression were evaluated in 30 tumor tissue samples of PTC by immunohistochemistry. Immunohistochemistry was performed on formalin-fixed, paraffin embedded, 3-μm-thick tissue sections. We have deparaffinized sections then dehydrated them using a graded ethanol. We have blocked endogenous peroxidase activity by incubation with methanol and 0.3 % hydrogen peroxidase for 20 minutes, then we rinsed sections in phosphate-buffered saline (PBS), processed tissue sections in a citrate buffer (pH 6.0) inside a plastic container which is heat resistant. Sections were put in a microwave oven for 20minutes. Then, the slides were cooled at room temperature. The primary antibodies that were used are; primary rabbit monoclonal anti- USP22 antibody (1:15, Atlas Antibodies, Sigma- Aldrich, USA) and anti STIP-1 antibody (EPR-6606, ab126753) dilution 1:200. We have incubated sections with the primary antibodies overnight at 4 °C then with the secondary antibody. We have used sections from colon cancer and ovarian-carcinoma sections as positive controls for USP22 and STIP-1 respectively, the negative controls are slides that were stained without primary antibody but replaced with non-immune PBS.
Evaluation of immunohistochemical expression of USP22 and STIP-1
The stained slides are evaluated by an expert pathologist who was blinded to clinic pathological data and outcomes of the cases.
Positive nuclear expression was considered positive for USP22 while positive cytoplasmic expression was considered positive for STIP-1.
USP22 and STIP-1 expression was quantified using a grading system which is based on the extent of stain which is percentage of tumor cells which are stained positive then graded them on a scale from 0 - 3: 0= negative stain in tumor cells, 1=1–30 % stain in tumor cells, 2=31–60 % stain in tumor cells, 3>60 % stain in tumor cells and the intensity of staining is graded on a scale from 0–3: 0=no stain, 1 = weak staining in tumor cells, 2=moderate staining in tumor cells, 3 = strong staining in tumor cells. Combination of stain extent and intensity was obtained by multiplying values of both of them to yield results from 0 to 9. Final scores from 0–3 were considered low expression of both markers and final scores >3 were considered high expression of both markers.14
Continuous variables were written as the median (range) and the mean ± SD, while categorical variables were written as a number (percentage). We have used Mann Whitney U test for non-normally distributed data and used Kraskall Wallis H test for comparison between more than 2 groups of non-normally distributed variables. Pearson’s Chi-square test was used for comparison between percent of categorical variables. Disease Free Survival (DFS) rate is the time from date of complete remission to date at which local, regional recurrence or distant metastasis was detected or to the most recent follow-up data. Overall Survival (OS) rate was calculated as the time from date of PTC diagnosis to date of death or the most recent follow-up data. Stratification of OS & DFS was according to studied clinic-pathological, USP22 and STIP-1 expressions using the method of Kaplan-Meier curve. A p-value of less than 0.05 was considered-significant significant. Statistics are done using SPSS 22.0 for windows (USA; Chicago, SPSS Inc.) and MedCalc-windows (MedCalc Software bvba 13, Ostend, Belgium).
Patients' demographic data are detailed in Table 1
Characteristics |
Number |
% |
Age (year) |
||
Median (Range) |
39.50 (28 – 56) |
|
<45 years |
20 |
58.3% |
≥45 years |
10 |
41.7% |
Sex |
||
Male |
8 |
27.8% |
Female |
22 |
72.2% |
Surgery |
||
Lobectomy |
1 |
2.8% |
Subtotal thyroidectomy |
5 |
19.4% |
Total thyroidectomy |
13 |
44.4% |
Total thyroidectomy+BND |
11 |
33.3% |
Histopathological subtype |
||
Conventional |
22 |
83.3% |
Follicular variant |
8 |
16.7% |
Tumour size (cm) |
||
Median (Range) |
3 (0.50 – 5) |
|
≤ 4 cm |
22 |
69.4% |
> 4 cm |
8 |
30.6% |
Multifocality |
||
Absent |
20 |
61.1% |
Present |
10 |
38.9% |
Capsular invasion |
||
Absent |
21 |
69.4% |
Present |
9 |
30.6% |
Extrathyroid extension |
||
Absent |
22 |
75% |
Present |
8 |
25% |
Vascular invasion |
||
Absent |
21 |
80.6% |
Present |
9 |
19.4% |
LN involvement |
||
Absent |
18 |
61.1% |
Present |
12 |
38.9% |
Distant metastasis |
||
Absent |
25 |
86.1% |
Present |
5 |
13.9% |
T |
||
T1 |
5 |
19.4% |
T2 |
15 |
47.2% |
T3 |
3 |
8.3% |
T4 |
7 |
25% |
N |
||
N0 |
19 |
61.1% |
N1 |
11 |
38.9% |
M |
||
M0 |
24 |
86.1% |
M1 |
6 |
13.9% |
Stage |
||
Stage I |
18 |
61.1% |
Stage II |
8 |
27.8% |
Stage III |
2 |
5.6% |
Stage IV |
2 |
5.6% |
Table 1 Clinicopathological features of papillary thyroid carcinoma patients
Continuous variables were expressed as mean ± SD & median (range); categorical variables were expressed as number (percentage)
USP22 protein expression in PTC tissue
The expression of USP22 protein in PTC tissue samples as analyzed by immunohistochemistry.
We proved that 60.6 % (18/30) of the PTC cases showed high USP22 expression.
Association of USP22 tissue protein expression with the clinicopathological features of PTC
We have observed that increased USP22 expression was associated with larger PTC size (p=0.02), presence of extra-capsular invasion (p=0.004), multi-focality (p=0.002), extrathyroidal extension (p=0.006), presence of lymph node metastasis (p=0.03), presence of vascular invasion (p=0.008), presence of distant metastasis (p=0.042), and advanced stage (p=0.012) in patients with PTC (Table 2). There was no significant association between USP22 expression, age, sex of our patients, and histopathological subtype of the PTC, type of performed surgery or dose of radioactive iodine used for the patients.
USP22 |
STIP-1 |
||||||
|---|---|---|---|---|---|---|---|
All(N=30) |
Low(N=12) |
High(N=18) |
p-value |
Low (N=14) |
High(N=16) |
p-value |
|
Characteristics |
No. (%) |
No. (%) |
No. (%) |
No. (%) |
No. (%) |
||
Age (years) |
|||||||
Median (Range) |
41.50 (21-53) |
45.50 (22-53) |
33 (21-50) |
43.50 (21-52) |
38 (22-50) |
||
<45 years |
20 (58.3%) |
9 (42.9%) |
11 (57.1%) |
0.080‡ |
7 (52.4%) |
13 (47.6%) |
0.202‡ |
≥45 years |
10 (41.7%) |
3 (73.3%) |
7 (26.7%) |
7 (73.3%) |
3 (26.7%) |
||
Sex |
|||||||
Male |
8 (27.8%) |
4 (60%) |
4 (40%) |
0.900‡ |
6 (60%) |
2 (40%) |
2.000‡ |
Female |
22 (72.2%) |
8 (53.8%) |
14 (46.2%) |
8 (61.5%) |
14 (38.5%) |
||
Surgery |
|||||||
Lobectomy |
1 (2.8%) |
0 (0%) |
1 (100%) |
0.190‡ |
0 (0%) |
1 (100%) |
0.322‡ |
Subtotal thyroidectomy |
5 (19.4%) |
1 (42.9%) |
4 (57.1%) |
4 (57.1%) |
1 (42.9%) |
||
Total thyroidectomy |
13 (44.4%) |
8 (50%) |
5 (50%) |
7 (56.3%) |
6 (43.8%) |
||
Total thyroidectomy+BND |
11 (33.3%) |
3 (75%) |
8 (25%) |
3 (75%) |
8 (25%) |
||
Histopathological subtype |
|||||||
Conventional |
22 (83.3%) |
12 (60%) |
10 (40%) |
0.264‡ |
12 (66.7%) |
10 (33.3%) |
0.162‡ |
Follicular variant |
8 (16.7%) |
3 (33.3%) |
5 (66.7%) |
2 (33.3%) |
6 (66.7%) |
||
Tumour size (cm) |
|||||||
≤ 4 cm |
20 (69.4%) |
9 (72%) |
11 (28%) |
0.02‡ |
10 (76%) |
10 (24%) |
0.030‡ |
> 4 cm |
10 (30.6%) |
3 (18.2%) |
7 (81.8%) |
4 (27.3%) |
6 (72.7%) |
||
Multifocality |
|||||||
Absent |
20 (61.1%) |
10 (81.8%) |
10 (18.2%) |
0.002‡ |
11 (86.4%) |
9 (13.6%) |
0.003‡ |
Present |
10 (38.9%) |
2 (14.3%) |
8 (85.7%) |
3 (21.4%) |
7 (78.6%) |
||
Capsular invasion |
|||||||
Absent |
21 (69.4%) |
11 (76%) |
10 (24%) |
0.004‡ |
12 (84%) |
9 (16%) |
0.004‡ |
Present |
9 (30.6%) |
1 (9.1%) |
8 (90.9%) |
2 (9.1%) |
7 (90.9%) |
||
Extrathyroid extension |
|||||||
Absent |
22 (75%) |
12 (74.1%) |
10 (25.9%) |
0.006‡ |
14 (81.5%) |
8 (18.5%) |
0.009‡ |
Present |
8 (25%) |
0 (0%) |
8 (100%) |
0 (0%) |
8 (100%) |
||
Vascular invasion |
|||||||
Absent |
21 (80.6%) |
12 (69%) |
9 (31%) |
0.008‡ |
14 (75.9%) |
7 (24.1%) |
0.002‡ |
Present |
9 (19.4%) |
0 (0%) |
9 (100%) |
0 (0%) |
9 (100%) |
||
LN involvement |
|||||||
Absent |
18 (61.1%) |
8 (81.8%) |
10 (18.2%) |
0.03‡ |
10 (81.8%) |
8 (18.2%) |
0.034‡ |
Present |
12 (38.9%) |
4 (14.3%) |
8 (85.7%) |
4 (28.6%) |
8 (71.4%) |
||
Distant metastasis |
|||||||
Absent |
25 (86.1%) |
12 (64.5%) |
13 (35.5%) |
0.042‡ |
14 (71%) |
9 (29%) |
0.006‡ |
Present |
5 (13.9%) |
0 (0%) |
5 (100%) |
0 (0%) |
5 (100%) |
||
T |
|||||||
T1 |
5 (19.4%) |
5 (100%) |
0 (0%) |
0.003§ |
5 (100%) |
0 (0%) |
0.008§ |
T2 |
15 (47.2%) |
7 (76.5%) |
8 (23.5%) |
8 (82.4%) |
7 (17.6%) |
||
T3 |
3 (8.3%) |
0 (0%) |
3 (100%) |
1 (33.3%) |
2 (66.7%) |
||
T4 |
7 (25%) |
0 (0%) |
7 (100%) |
0 (0%) |
7 (100%) |
||
N |
|||||||
N0 |
19 (61.1%) |
9 (81.8%) |
10 (18.2%) |
0.007‡ |
11 (81.8%) |
8 (18.2%) |
0.02‡ |
N1 |
11 (38.9%) |
3 (14.3%) |
8 (85.7%) |
3 (28.6%) |
8 (71.4%) |
||
M |
|||||||
M0 |
24 (86.1%) |
12 (64.5%) |
12 (35.5%) |
0.031‡ |
14 (71%) |
10 (29%) |
0.001‡ |
M1 |
6 (13.9%) |
0 (0%) |
6 (100%) |
0 (0%) |
6 (100%) |
||
Stage |
|||||||
Stage I |
18 (61.1%) |
8 (63.6%) |
10 (36.4%) |
0.012§ |
12 (72.7%) |
2 (27.3%) |
0.018§ |
Stage II |
8 (27.8%) |
4 (60%) |
4 (40%) |
2 (60%) |
6 (40%) |
||
Stage III |
2 (5.6%) |
0 (0%) |
2 (100%) |
0 (0%) |
2 (100%) |
||
Stage IV |
2 (5.6%) |
0 (0%) |
2 (100%) |
0 (0%) |
2 (100%) |
||
USP22 |
|||||||
Low |
12 (55.6%) |
12 (100%) |
0 (0%) |
0.01‡ |
|||
High |
18 (44.4%) |
2 (12.5%) |
16 (87.5%) |
||||
STIP-1 |
|||||||
Low |
14 (61.1%) |
12 (90.9%) |
2 (9.1%) |
0.01‡ |
|||
High |
16 (38.9%) |
0 (0%) |
16 (100%) |
||||
Radioiodine therapy dose(mCi) |
|||||||
Mean ± SD |
197.50 ±183.04 |
195 ±207.12 |
200.62 ±154.33 |
0.628• |
184.54 ±199.89 |
217.85 ±157.87 |
0.343• |
Median (Range) |
100 (60-870) |
105 (60-870) |
100 (80-540) |
100 (60-870) |
110 (80-540) |
||
Table 2 Associations of clinicopathological features and immunohistochemical markers in papillary thyroid carcinoma patients
* Independent samples Student's t-test; • Mann Whitney U test; ‡ Chi-square test; § Chi-square test for trend; p<0.05 is significant
Survival analysis
To detect the prognostic role of USP22 in PTC, we evaluated the association between its expression and patients' survival rates by using a Kaplan–Meier method. We have observed that increased USP22 expression was associated with higher incidence of loco-regional recurrence (LRR), neck node recurrence (p=0.26 and 0.003 respectively). We have found that PTC patients with high USP22 expression had a shorter OS & DFS when compared to patients with low USP22 expression (p=0.014 and 0.006 respectively). There was no significant association between USP22 expression, tumor bed recurrence or mediaFstinal recurrence. The results showed that tissue protein expression of USP22 was an independent prognostic factor for PTC patients.
STIP-1 protein expression in PTC tissue
The expression of STIP-1 protein in PTC tissue samples as analyzed by immunohistochemistry.
We proved that 58.6 % (16/30) of the PTC cases showed high STIP-1 expression.
Association of STIP-1 tissue protein expression with the clinicopathological features of PTC
We have observed that increased STIP-1 expression was associated with larger PTC size (p=0.03), presence of extra-capsular invasion (p=0.004), multi-focality (p=0.003), extrathyroidal extension (p=0.009), presence of lymph node metastasis (p=0.034), presence of vascular invasion (p=0.002), presence of distant metastasis (p=0.006), and advanced stage (p=0.018) in patients with PTC. There was no significant association between STIP-1 expression, age, sex of our patients, and histopathological subtype of the PTC, dose of radioactive iodine used for the patients or type of performed surgery.
Survival analysis
To detect the prognostic role of STIP-1 in PTC, we evaluated the association between its expression and patients' survival rates by using a Kaplan–Meier method. We have found that PTC patients with high STIP-1 expression had a shorter OS & DFS when compared to patients with low STIP-1 expression (p=0.014 and 0.043 respectively). There was no significant association between STIP-1 expression, tumor bed recurrence or mediastinal recurrence, loco-regional recurrence (LRR) or neck node recurrence (Table 3).
USP22 |
STIP-1 |
||||||
|---|---|---|---|---|---|---|---|
All(N=30) |
Low(N=12) |
High(N=18) |
p-value |
Low (N=14) |
High(N=16) |
p-value |
|
Outcome |
No. (%) |
No. (%) |
No. (%) |
No. (%) |
No. (%) |
||
Response to treatment |
|||||||
SD |
1 (2.8%) |
1 (5%) |
0 (0%) |
0.657‡ |
1 (4.5%) |
0 (0%) |
0.689‡ |
PR |
2 (5.6%) |
1 (5%) |
1 (6.3%) |
1 (4.5%) |
1 (7.1%) |
||
CR |
27 (91.7%) |
10 (90%) |
17 (93.8%) |
12 (90.9%) |
15 (92.9%) |
||
NR |
3 (8.3%) |
2 (10%) |
1 (6.3%) |
1.000‡ |
2 (9.1%) |
1 (7.1%) |
1.000‡ |
OAR |
27 (91.7%) |
10 (90%) |
17 (93.8%) |
12 (90.9%) |
15 (92.9%) |
||
Time to Complete remission |
|||||||
Mean ± SD |
14.51 ± 4.77 |
14.94 ±4.19 |
14 ±5.50 |
0.841• |
15.10 ±3.99 |
13.61 ±5.83 |
0.414• |
Median (Range) |
13 (4-29) |
14 (10-24) |
13 (4-29) |
15 (10-24) |
13 (4-29) |
||
Post-ablation TG |
|||||||
Mean ± SD |
23.28 ± 126.37 |
0.98 ±3.07 |
51.16 ±189.10 |
0.080• |
0.91 ±2.93 |
58.44 ±202.01 |
0.033• |
Median (Range) |
0.40 (0.1-759) |
0.20 (0.1-14) |
0.45 (0.1-759) |
0.20 (0.1-14) |
0.50 (0.1-759) |
||
Mortality |
|||||||
Absent |
23 (80.6%) |
9 (85%) |
14 (75%) |
0.048‡ |
11 (86.4%) |
12 (71.4%) |
0.0494‡ |
Present |
7 (19.4%) |
3 (15%) |
4 (25%) |
3 (13.6%) |
4 (28.6%) |
||
Relapse |
|||||||
Absent |
12 (33.3%) |
6 (45%) |
6 (18.8%) |
0.044‡ |
9 (40.9%) |
3 (21.4%) |
0.039‡ |
Present |
18 (58.3%) |
6 (45%) |
12 (75%) |
5 (50%) |
13 (71.4%) |
||
Events |
|||||||
No |
10 (33.3%) |
6 (45%) |
4 (18.8%) |
0.024‡ |
4 (40.9%) |
6 (21.4%) |
0.021‡ |
Local recurrence (LR) |
15 (50%) |
5 (40%) |
10 (62.5%) |
8 (45.5%) |
7 (57.1%) |
||
Distant metastasis (DM) |
2 (8.3%) |
1 (10%) |
1 (6.3%) |
2 (9.1%) |
1 (7.1%) |
||
LR + DM |
1 (2.8%) |
0 (0%) |
1 (6.3%) |
0 (0%) |
1 (7.1%) |
||
LRR |
|||||||
Absent |
11 (38.9%) |
10 (50%) |
1 (25%) |
0.026‡ |
9 (45.5%) |
2 (28.6%) |
0.538‡ |
Present |
19 (52.8%) |
2 (40%) |
17 (68.8%) |
5 (45.5%) |
12 (64.3%) |
||
Tumor bed recurrence |
|||||||
Absent |
17 (63.9%) |
9 (65%) |
8 (62.5%) |
0.868‡ |
10 (68.2%0 |
7 (57.1%) |
0.697‡ |
Present |
10 (27.8%) |
3 (25%) |
7 (31.3%) |
4 (22.7%) |
6 (35.7%) |
||
Neck node recurrence |
|||||||
Absent |
17 (63.9%) |
16 (85%) |
4 (37.5%) |
0.003‡ |
13 (77.3%) |
6 (42.9%) |
0.058 |
Present |
10 (27.8%) |
1 (5%) |
8 (56.3%) |
3 (13.6%) |
7 (50%) |
||
Mediastinal recurrence |
|||||||
Absent |
27 (88.9%) |
13 (90%) |
14 (87.5%) |
0.495‡ |
20 (90.9%) |
12 (85.7%) |
0.441‡ |
Present |
1 (2.8%) |
0 (0%) |
1 (6.3%) |
0 (0%) |
1 (7.1%) |
||
Distant metastasis |
|||||||
Absent |
26 (86.1%) |
10 (90%) |
16 (81.3%) |
0.502‡ |
10 (90.9%) |
11 (78.6%) |
0.381‡ |
Present |
4 (11.1%) |
2 (10%) |
2 (12.5%) |
4 (9.1%) |
2 (14.3%) |
||
OS |
|||||||
Mean (month) |
59.7 months |
59.9 months |
59.4 months |
0.014† |
59.9 months |
59.4 months |
0.014† |
(95%CI) |
(59.4-60.1) |
(58.7-60.2) |
(59.6-60.2) |
(58.7-60.2) |
|||
HR (95%CI) |
---- |
4.825 (1.051 – 22.148) |
4.825 (1.051 – 22.148) |
||||
24 month OS (%) |
100% |
100% |
100% |
100% |
100% |
||
36 month OS (%) |
100% |
100% |
100% |
100% |
100% |
||
48 month OS (%) |
100% |
100% |
100% |
100% |
100% |
||
60 month OS (%) |
57.9% |
75% |
0% |
75% |
0% |
||
DFS |
|||||||
Mean (month) |
52.9 months |
55.3 months |
49.9 months |
0.006† |
53.9 months |
51.2 months |
0.043† |
(95%CI) |
(49.3-56.5) |
(50.2-60.4) |
(45.1-54.7) |
(48.8-59.1) |
(46.6-55.7) |
||
HR (95%CI) |
---- |
3.241 (1.308 – 8.029) |
2.370 (0.975 – 5.761) |
||||
24 month PFS (%) |
97% |
94.4% |
100% |
95% |
100% |
||
36 month PFS (%) |
93.9% |
94.4% |
93.3% |
90% |
100% |
||
48 month PFS (%) |
75.8% |
83.3% |
66.7% |
80% |
69.2% |
||
60 month PFS (%) |
29.6% |
45.8% |
0% |
41.30% |
0% |
||
Table 3 Relation between immunohistochemical markers and outcome of papillary thyroid carcinoma patients
† Log rank test; HR, Hazards Ratio; 95%CI: 95% Confidence Interval; p< 0.05 is significant
The results showed that tissue protein expression of STIP-1 was an independent prognostic factor for PTC patients. Expressions of both USP22 and STIP-1 were significantly positively correlated with each other (p<0.001) (Figure 1‒4).
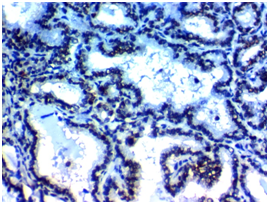
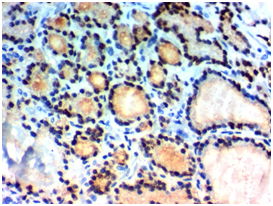
Figure 1A Figure 1B
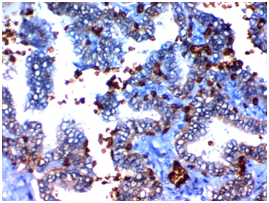
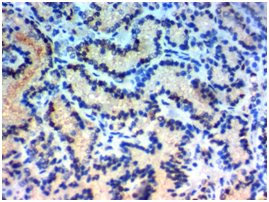
Figure 1C Figure 1D
Figure 1 USP22 expression in Papillary thyroid carcinoma (PTC): (A) High nuclear expression of USP22 in conventional PTC high grade stage IVx400. (B) High nuclear expression of USP22 in PTC follicular variant high grade, stage IIIx400. (C) Low nuclear expression of USP22 in conventional PTC low grade, stage IIBx400. (D) Low nuclear expression of USP22 in PTC follicular variant low grade; stage IIBx400.


Figure 2A Figure 2B
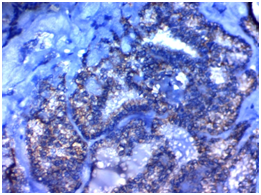
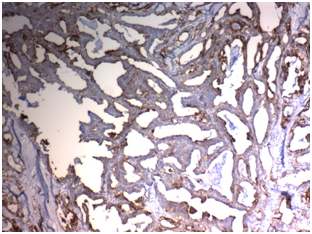
Figure 2C Figure 2D
Figure 2 STIP1expression in Papillary thyroid carcinoma (PTC): (A) High cytoplasmic expression of STIP1in conventional PTC high grade stage IVx400. (B) High cytoplasmic expression of STIP1in PTC follicular variant high grade, stage IIIx200. (C) Low cytoplasmic expression of STIP1in conventional PTC low grade, stage IIBx400. (D) Low cytoplasmic expression of STIP1in PTC follicular variant low grade; stage IIBx200.
Cancer epigenetics field is considered now a novel point of cancer research.15 Histone modifications play an essential role in tumorigenesis, cancer diagnosis and cancer patients' follow-up.16 Ubiquitination which is one of the histone modifications could be involved in transcription, repair of DNA damage and meiosis.15 The recently detected deubiquitinating enzyme; USP22, is considered a cancer stem cell marker which has ubiquitin hydrolase activity.14
USP22 could be able to cleave ubiquitin efficiently and it was a marker gene which is capable of predicting metastatic potential and high liability of therapy failure in human cancer.3,5,6,16 Moreover USP22 is incriminated in carcinogenesis process and cell cycle progression in malignant cells. It was proved that USP22 contributed to tumor cells growth, while its down-regulation induced cell cycle arrest in non-small cell lung carcinoma and bladder cancer cells,4,17 these results signified that USP22 depletion resulted in cell cycle arrest at the G1 phase, which leads to reduction in the S and G2/M phases. Additionally, USP22 recruitment leads to transcriptional activation of essential cell cycle genes that have roles in cell cycle progression.4,18 The role of USP22 has been studied in many organs but its detailed role has not been clarified in PTC so in the current study we tried to detect tissue protein expression of such biomarker in PTC and detect its pathological and clinical values.
We have proved that USP22 is overexpressed in PTC tissue and its expression was related to higher grade higher liability for invasion of nearby structures, larger tumor size, presence of extra-capsular invasion, multi-focality higher incidence of lymphatic and hematogenous spread. Also during follow-up of our patients we have found that high USP expression is associated with higher incidence of recurrence of the tumor, poor response to therapy and unfavorable survival rates. In the Kaplan–Meier survival curves analysis, OS rate of patients with high USP22 expression in their tumor tissues was shorter than that of patients having low USP22 expression.
Our results were similar to results of Zhao et al.,15 and Wang et al.,14 who proved the same association in PTC patients, additionally, Zhao et al.,15 have proved that targeted therapy against USP22 decreased cell cycle progression in cancer cells and improve patients prognosis.
Similarly, Li et al.,19 in colon cancer have declared the association between USP expression and poor patient prognosis, and they have found that USP22 promotes CRC metastasis, migration and invasion capacities. Li et al.,19 have explained their results by that USP22 which is an up-stream molecule of AP4, exhibits the potential to increase CRC migration and invasion capacities, by inducing EMT by activating AP4. Moreover, USP22 adversely affect OS in CRC patients. Moreover, Dai et al.,20 proved the same expression pattern and clinical significance of USP22 in salivary adenoid cystic carcinoma (SACC) and they have found that USP22 high expression was associated with unfavorable histological subtypes, presence of lymph node metastasis, higher grade, higher Ki-67 labeling index and patterns of expression of USP22 could be a predictive factor of SACC as they have found that SACC patients with high USP22 expression had low OS and DFS rates. Also, Piao et al.,21 also showed that higher expression levels of USP22 in tissues of salivary duct carcinoma were associated with a poor patients' prognosis.
As Wang et al.,14 found that levels of PTC tissue expression of both USP22 mRNA and protein were higher than those in benign thyroid tissues, so that USP22 might be considered an oncogene that is responsible for carcinogenesis and progression of PTC. All these results suggested that the increased USP22 expression might allow identification of PTC patients with a poor prognosis thus it could be considered a novel prognostic marker for patients with PTC. Dai et al.,20 have detected that USP22 is highly expressed in malignant tissues and is associated with progression and unfavorable clinical outcome. These results were explained by that USP22 allows cell-cycle progression through regulation of INK4a/ARF and Akt signaling pathways. Moreover, Xu et al.,22 have revealed that USP22 knocking down by micro-RNA interference could be able to inhibit growth and invasion of colorectal cancer.
Our results suggested that high tissue protein USP22 expression could be able to expect a poor prognosis in PTC patients, and the suppression of its expression might provide a target for therapeutic intervention in PTC patients. We have studied another cell cycle marker in PTC tissues that was also incriminated in cell cycle progression in malignant cells that is STIP-1 In our research, we proved that STIP-1 is over expressed in PTC tissue and its expression was related to higher grade higher liability for invasion of nearby structures, larger tumor size, presence of extra-capsular invasion, multi-focality higher incidence of lymphatic and hematogenous spread. Also during follow-up of our patients we have found that high STIP-1 expression is associated with higher incidence of recurrence of the tumor, poor response to therapy and unfavorable survival rates. In the Kaplan–Meier survival curves analysis, OS rate of patients with high STIP-1expression in their tumor tissues was shorter than that of patients having low STIP-1 expression.
So STIP-1 expression was associated with unfavorable prognosis. Results of the current study was similar to results of Yuan et al.,23 and Tsai et al.,24 who have reported that STIP-1 over expression in PTC tissues is related to higher incidence of occurrence of lymph nodes metastasis, larger cancer size, and advanced TNM-stages, so STIP-1 expression is related to dismal prognosis and it could be considered a novel prognostic biomarker for PTC patients outcome.
Also our results were similar to results of Huang et al.,25 which proved that Stress-inducible Protein-1 promotes gastric cancer cells invasion and metastasis via Wnt/β-catenin signaling pathway and by down regulation of E-cadherin and other epithelial markers which could induce epithelial mesenchymal process which is responsible for malignant cells invasion and metastases. Moreover Zhang et al.,26 proved similar results in colon cancer cells High STIP1 expression was correlated with advanced stage and higher grade. Moreover, Kaplan-Meier analyses indicated that higher STIP1 expression predicted a worse prognosis in patients with CRC, and Cox regression analysis revealed that STIP1 was an independent prognostic factor for overall survival and disease-free survival in patients with CRC. So they have proved that STIP1 acts as an oncogene in CRC and can be considered a biomarker for the prognosis of patients with CRC.
Higher tissue protein expression of STIP-1 is associated with unfavorable prognosis in cancers of many organs as hepatocellular carcinoma, gliomas, pancreatic, ovarian cancer and cholangiocarcinoma,27‒31 these results are explained by that this bio-marker is considered anti-apoptotic factor which could increase malignant cells growth and survival. Moreover it was found that effective inhibition of STIP-1 over expressed cancer cell leads to inhibition of cells proliferation and migration that have been accomplished by targeting STIP-1 by specific anti-STIP-1 antibodies and those antibodies are novel therapeutic targets for management of ovarian, pancreatic, lung, prostate, renal, gastric cancers and melanoma.32
To best of our knowledge no previous studies have assessed tissue protein expression of both USP22 and STIP-1 in PTC. In our study we have found a significant positive correlations between both of them as both markers were incriminated in cell cycle progression in PTC which is associated with higher incidence of invasion metastases and dismal patients' outcome. Targeting both markers could be considered novel therapy for PTC which improves its prognosis in addition to the currently used therapy.
Results of the current study have shown that increased USP22& STIP-1 expression in PTC is positively correlated with poor clinical and pathological parameters in addition to association with dismal patients' outcome. Furthermore, their levels of expression are independent predictors of survival for PTC patients. To date, many previous studies found that USP22& STIP-1 represent novel prognostic biomarkers in PTC progression and oncogenesis, but the molecular mechanism of association of increased their expression and cancer metastasis is still unknown. Further future studies and more samples is required for more clarification of their role in cancer progression, invasion and metastases. So both USP22 & STIP-1 might be considered attractive and promising therapeutic targets for PTC patients.
None.
The author declares that they have no competing interests.

©2018 Fouad, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.