Advances in
eISSN: 2573-2862


Research Article Volume 3 Issue 3
1Department of Pathology, Montefiore Medical Center, USA
2Department of Medicine, Nyack Hospital, USA
3Department of Pathology, NYU Langone Health, USA
Correspondence: Ding Wen Wu, NYU Langone Hospital-Brooklyn, 150 55th Street, Brooklyn, NY 11220, USA, Tel 646-899-5173
Received: August 02, 2018 | Published: August 20, 2018
Citation: Himchak EL, Friedmann R, Himchak S, et al. Optimizing hemoglobin s percentage for pediatric sickle cell disease patients chronically transfused with red cell exchange. Adv Cytol Pathol. 2018;3(3):68-72 DOI: 10.15406/acp.2018.03.00056
In patients with Sickle Cell Disease (SCD), transfusion therapies have shown to reduce the risk of strokes in patients with abnormal cerebral blood flow. The current guidelines for pediatric patients with SCD recommend monthly transfusions using red cell exchange (RCE) to maintain the pediatric patient’s sickle hemoglobin (HbS) below 30% between treatments. However, it is not clear how much RCE is needed to achieve this goal. Our objective was to find a target post-RCE HbS (post-HbS) level, following which our patients’ HbS would remain below 30% between monthly RCE. We identified RCE events for pediatric patients = 20 years old with the HbSS genotype and receiving monthly RCE therapy. HbS results were categorized into post-HbS and follow-up RCE HbS (F/u-HbS) from twenty two to forty days later. Two hundred and seventy two complete sets of data from 14 patients were identified from 2000 to 2015. No particular level of post-HbS accurately predicts the exact level of F/u-HbS. Of patients with post-HbS of 5-10%, 67% had F/u-HbS < 30%. Additionally only 20% of patients with post-HbS of 10-15% had F/u-HbS < 30%. Reduction of post-HbS to 5-10% was found to be the most effective in maintaining the HbS level < 30% between monthly RCE.
Keywords: sickle cell anemia, red cell exchange, hbs, pediatrics hbss
Patients with Sickle Cell Anemia (SCA), genotype HbSS, have abnormal β-globin chains of the hemoglobin molecule. When deoxygenated, these abnormal RBCs form insoluble polymers which cause deformation of red blood cells to a “sickle” shape.1 This change causes clinical consequences from a series of complex mechanisms.2 One of the critical events is vascular abnormalities, including hemolysis, inflammation, vascular adhesion, vaso-occlusion, thrombosis, ischemia, hypoxia, etc. Early intervention can reduce or, at least, delay many of the complications seen in SCA.3
It is estimated that 11% of patients with SCA experience a stroke before the age of 20, and that figure increases to 24% by the age of 45 years.4,5 In some pediatric patients, cerebrovascular abnormalities were partially healed or even reversed.6 Similarly, Russel et al.,6 documented a stabilization of arterial disease and stenosis in transfused patients.7 In fact, studies have shown that chronic transfusions decrease the amount of HbS and reduce the risk of serious vascular complications such as ischemic stroke in patients with SCA.8
Chronic transfusion therapy is indicated for stroke prevention. Without therapy, initial strokes occur in 60-90% of patients.6 The Stroke Prevention Trial in Sickle Cell Anemia showed a 90% decline in stroke rate in children with an abnormal transcranial doppler who kept HbS < 30%.8?10 Russel et al.6 noted that with transfusion, the percentage of stroke recurrence decreased dramatically from 90% to 10%.7 Pegelow et al.11 suggested that maintenance of HbS < 30% significantly reduces the risk of stroke recurrence in pediatric patients.11 Moreover, Yawn BP et al.12 recommend keeping HbS levels below 30% in all long-term transfused SCA children to prevent associated risks, not just for stroke prevention.11
Red cell exchange (RCE) is considered an effective therapy for both acute and non-acute complications of SCA.13?15 Modern apheresis machines can be used to achieve a specific target post-RCE HbS (post-HbS). The machines either have built-in formulae or web-based tools which are used to determine how many units of RBCs to use for RCE in order to reach the predetermined post-HbS level.16 Measurement of pre-RCE HbS (pre-HbS) and post-HbS levels can be used to improve the efficacy of the RCE. Unless a patient requires an increased hematocrit for surgery or acute indications, the machine is often set to produce a post-RCE hematocrit level close to the patient’s baseline level.17
Automated red cell exchange transfusion, not manual exchange transfusion, further improves blood viscosity to increase oxygen delivery for SCA patients. This might be due to the control of hematocrit allowed by the automated procedure, while decreasing the HbS percentage.18
DeBaun et al.19 suggested lowering post-HbS into the range of 15-20% for patients with acute organ deterioration. The authors suggest that it is unlikely for the HbS to increase above 30% within three weeks, despite lack of evidence. They also believe that this would minimize complications caused by the sickled RBCs. Evidence supporting that reaching this range post-transfusion is more advantageous than 30% is needed.19
To comply with the current guidelines for pediatric patients with SCA,12 post-HbS 10-15% and/ or 15-20% are often empirically utilized in the efforts of maintaining HbS < 30% in the following month (unpublished communication).
Many authors have discussed lowering HbS to < 30%, yet data is lacking for how to keep the HbS levels below 30% between RCEs. We retrospectively analyzed our RCE data spanning 15 years, to determine a target post- HbS level which would result in maintenance of HbS below 30% between RCE therapies. The results from this study will shed light on optimizing pediatric RCE for HbSS, and potentiating compliance with the current standard of care. To our knowledge, this is the first study which aims to determine a possible approach to effectively keep HbS levels remain < 30% between RCE therapies.
The aim of present study is to determine a reliable approach to keep HbS levels remain < 30% between RCE therapies, in order to be compliant with the current treatment guidelines for pediatric SCA patients, and prevent initial and recurrent strokes.
Red cell exchange
The COBE Spectra device and Spectra Optia (TerumoBCT, Lakewood, CO, USA) were used for all RCE procedures. For each procedure, a target level for both final hematocrit and fraction of cells remaining (FCR) was established based on the pre-hematocrit and pre-HbS. Using these targets, the volume of red cell exchanged was determined. In accordance with our institution’s policy, we used red cells which were phenotypically matched for the C, E, and K antigens. Additional phenotypic matching was done for patients with unexpected red cell antibodies. Anticoagulation was achieved with acid citrate dextrose A (ACD-A). The average hematocrit of the replacement RBCs was 57%. Pre- and post-HbS levels, and pre- and post- hematocrit (pre- and post-HCT) levels were obtained for each procedure.
Data criteria
We identified all the monthly RCE events for pediatric patients from January 2000 to May 2015, by using our institution’s patented clinical information system search tool Clinical Looking Glass (CLG). In this study we only included data from pediatric patients who met the following criteria: genotype HbSS, ≤ 20 years old, receiving monthly RCE treatments, and presenting with documented pre-HbS, post-HbS, and F/u-HbS, with the latter two separated by approximately one month (31± 9 days).
The following were used as exclusionary criteria: events with incomplete data points, data from patients with simple transfusions, and data from patients with other hemoglobinopathies.
Categorization
We extracted HbS results from these events and categorized them into post-HbS and F/u-HbS from 31 ± 9 days later. (In this retrospective study, considering patients’ compliance, ± 9 days of interval will be accepted.) Each set of data was considered as a patient event and arbitrarily categorized by the post-HbS of 5-10%, >10-15%, >15-20%, >20-25%, >25-30%, and > 30%, in order to find out the impact of different post-HbS levels on the F/u-HbS levels. Follow-up data was analyzed to identify if F/u-HbS was below 30%, 40%, and 50%.
We consider post-HbS< 5% an impractical goal, because none of our patients achieved post-HbS < 5%. A much increased number of RBC units are required to lower the post-HbS to < 5% given a pre-HbS > 30%.
Statistics
Using events from some of the individual patients, we constructed plots correlating post-HbS and F/u-HbS levels, to demonstrate variable inter-patient and intra-patient RCE response. Fischer’s Exact test was applied to F/u- HbS related to various categories of post-HbS. The result suggests statistically significant difference of F/u- HbS between paired post-HbS categories.
Comorbidities search
CLG was utilized to search the patients’ comorbidities before and during monthly RCE therapy.
Demographics
Through our longitudinal retrospective analysis over a 15-year period, we identified 486 RCE procedures performed for the pediatric patients. Among them, 272 RCE events from 14 patients met the inclusion criteria. Patient demographics and comorbidities before starting RCE monthly therapy are displayed in Table 1.
Patient # |
Sex |
History of Comorbidities Prior to Monthly RCE Regimen |
1 |
F |
LE ulceration, iron overload, arthralgia |
2 |
M |
Moyamoya, stroke |
3 |
F |
Moyamoya, stroke, hepatomegaly, cholecystectomy, pneumonia, TIA x2, chronic left MCA aneurysm, LICA and ACA aneurysm |
4 |
F |
Moyamoya, stroke, DVT, AVN R shoulder, somatization admitted for VOC |
5 |
M |
N/A |
6 |
M |
Moyamoya, stroke x2, seizure disorder, AVN right knee, proteinuria, Iron overload, priapism, hepatomegaly, cardiomegaly, splenic infarction |
7 |
M |
Stroke with right hemiparesis |
8 |
M |
Stroke, cholecystectomy, autosplenectomy |
9 |
M |
Moyamoya, stroke, iron overload, proteinuria, gall stones, laparoscopic cholecystectomy, LA and LV dilation |
10 |
F |
Moyamoya, proteinuria |
11 |
M |
Total splenectomy, cholecystectomy |
12 |
F |
Left hip Perthes disease, AVN femoral head |
13 |
M |
Moyamoya, right visual loss, priapism, acute chest syndrome x3 |
14 |
F |
Silent stroke, sickle cell crisis, asthma, acute chest syndrome, pain crisis |
Table 1 Patient demographics and pertinent comorbidities prior to receiving RCE therapy
LE, Lower extremity; TIA, Transient Ischemic Attach; MCA, Middle Cerebral Artery; LICA, Left Internal
Cerebral Artery; ACA, Anterior Cerebral Artery; DVT, Deep Vein Thrombosis; AVN, Avascular Necrosis;
VOC, Vaso-Occlusive Crisis; N/A, Not Applicable; LA, Left Atrium; LV, Left Ventricle;
Post- HbS and F/u-HbS
The acceptable F/u-HbS (< 30%) was achieved mostly with the post-HbS 5-10% group. 67% of patient events in the post-HbS 5-10%, category (n=9) had F/u-HbS < 30%, while 20% of patient events in the 10-15% category (n=25) had F/u-HbS < 30%. Few patient events with post-HbS > 15% had acceptable F/u-HbS (i.e. < 30%) results (9 of 272 patient events). When a patient’s post-HbS was = 20% by RCE, 0% had F/u-HbS < 30% one month later. In = 78% of these patient events, patients’ F/u-HbS exceeded 40% (Table 2).
Post-HbS (%) |
# of Post-HbS |
F/u-HbS < 30% (%) |
F/u-HbS < 40% (%) |
F/u-HbS < 50%(%) |
5-10 |
9 |
67 |
78 |
100 |
>10-15 |
25 |
20 |
72 |
96 |
>15-20 |
47 |
9 |
53 |
96 |
>20-25 |
108 |
0 |
22 |
87 |
>25-30 |
61 |
0 |
8 |
72 |
>30 |
22 |
0 |
0 |
50 |
Table 2 Correlation of Post-HbS % Level and F/u-HbS % Level (n=272)
Statistical trends and significance
Our focus is to find a cutoff value from the desired RCE targets to keep F/u-HbS < 30%. The particular level of post-HbS guarantees maintenance of F/u-HbS < 30%. Applying Fischer’s Exact test, it suggests statistically significant differences between the following paired groups: 5-10% and 10-15% (p=0.03), 5-10% and 15-20% (p=0.0004), and 5-10% and >10% (p=0.001). This test also suggests a lack of significance between the groups 10-15% and 15-20% (p=0.26) (Table 3).
Post HbS Group 1 |
Post HbS Group 2 |
p-value |
5-10% |
10-15% |
0.0328* |
5-10% |
15-20% |
0.0004* |
10-15% |
15-20% |
0.2602 |
5-10% |
> 10% |
0.001* |
Table 3 Significance when comparing levels of F/u-HbS related to various post-HbS categories
Variable outcomes
For each patient, monthly F/u-HbS values varied markedly over the course of years without a discernable pattern demonstrated by pivot analysis (data not shown). As displayed in the line-graph, variable F/u-HbS levels were observed between patients when post-HbS levels were the same (Figure 1(a-e)). Surprisingly, within an individual patient, levels vary significantly as well. For example, one patient had three instances of a post-HbS level of 24%, but the F/u-HbS levels were 45.1%, 37.8%, and 41.7% (Figure 1(a)). This same patient had two post-HbS levels of 26.9%, but a large difference between F/u-HbS, 47.3% and 38.6%. With large intra-patient variability, one cannot expect to observe consistent follow up HbS levels between several patients following the same post- HbS.
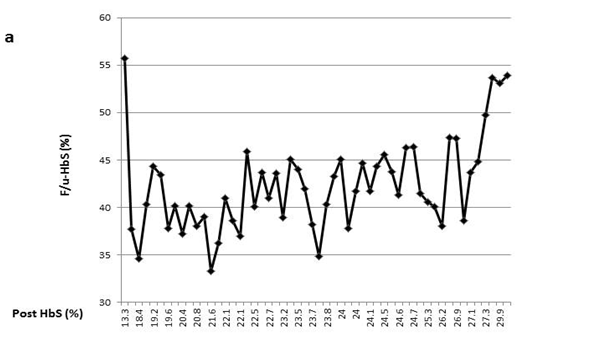
Figure 1(a) One patient had three instances of a post-HbS level of 24%, but the F/u-HbS levels were 45.1%, 37.8%, and 41.7%.
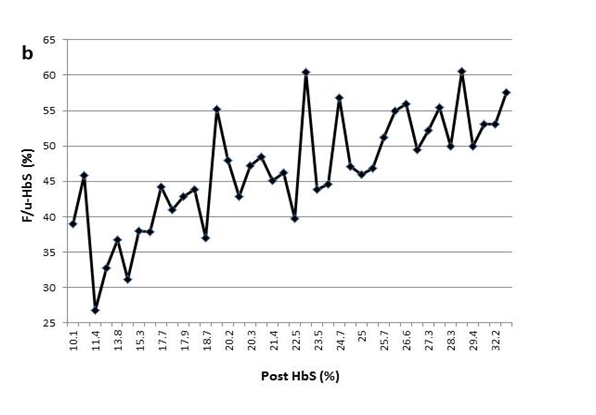
Figure 1(b) The variable F/u-HbS levels were observed in patient2 when post-HbS levels were the same.
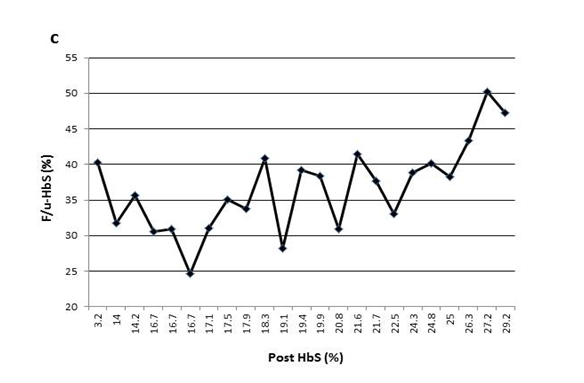
Figure 1(c) The variable F/u-HbS levels were observed in patient3 when post-HbS levels were the same.
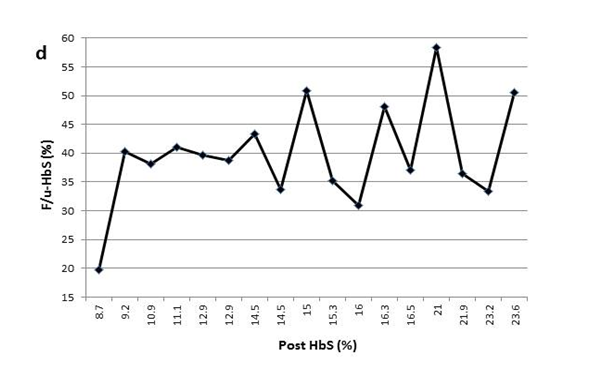
Figure 1(d) The variable F/u-HbS levels were observed in patient4 when post-HbS levels were the same.
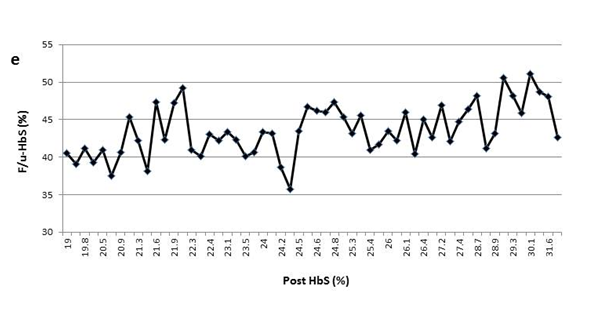
Figure 1(e) The variable F/u-HbS levels were observed in patient5 when post-HbS levels were the same.
Comorbidities during monthly RCE therapy
A CLG data search showed that patients did not experience comorbidities such as stroke or acute chest syndrome while receiving monthly RCE therapy.
Red cell exchange
One of the prominent benefits of RCE is that it can be programmed to reduce HbS from any pre-RCE level to desired targets of post-HbS and post-HCT level. That is, post-HbS and post-HCT can be easily achieved through RCE manipulation. However the F/u-HbS after each RCE cannot be simply achieved by manipulation. Fortunately, our study does reveal an effective way to help pediatric hematologists and apheresis specialists keep most of the F/u-HbS under 30% to meet the challenge of a new pediatric guideline for SCA patients.
HbS < 30% guideline
While HbS < 30% is the most commonly quoted pediatric guideline for SCA patients,17 evidence showed that lowering hemoglobin S to below 40% lowers viscosity.20 Cohen et al.21 found that a target HbS level of 50% afforded a high rate of protection, even against stroke, while reducing blood requirements significantly, and thereby lessening the rate of iron accumulation from simple transfusions.21 However, with a post HbS of 30%, it is uncertain if HbS would be kept consistently below 30%, 40% or 50% in the following month of the RCE. In fact, we would suggest that the possibility is unlikely because none of our patients whose post-HbS was between 25-30% had F/u-HbS < 30%, and more strikingly, only 8% of these patients had a F/u-HbS < 40%, and 72% of the patients had a F/u-HbS < 50%.
Keeping F/u-HbS < 30%, instead of < 50%, which was recommended in the guidelines for pediatric SCA patients, presents a big challenge. In our study, the acceptable F/u-HbS (< 30%) was achieved mostly with the post-HbS 5-10% group. None of post-HbS 20-30% resulted in F/u-HbS < 30%. Furthermore, not all the RCE events with post-HbS 20-30% could even keep F/u-HbS < 50%. Only 29% of RCE events with post-HbS 10-20% kept F/u-HbS < 30%. F/u-HbS < 30% was only achieved in the events of post-HbS 5-10%. Therefore, targeting post-HbS < 30% does not comply with current pediatric treatment guideline for SCA patients.
This process is further complicated by the fact that a specific post-HbS does not accurately predict a particular F/u-HbS level. Figure 1 shows examples of variation of the F/u-HbS in five patients. The examples demonstrate the variable results between patients who had similar or the same post-HbS levels. Additionally, this inconsistency is observed within the same patient whose HbS was lowered to the same level a few times.
Non-patterned F/u-HbS levels
Why is there inter-patient variability? In Zinkham et al.’s22 study regarding the amount of blood necessary for a simple transfusion, they observed differences in the subsequent pre-transfusion HbS percentages for patients who received the same amount of blood. Hoping to explain this discrepancy, they looked for differences in the reticulocytes, serum chemistry levels, erythropoietin, erythrocyte alloantibodies, and Howell-Jolly Bodies, but the data was inconclusive.22
In addition, we considered several factors that may have contributed to the discrepancy between patients F/u-HbS values despite having the same post-HbS levels. Fetal hemoglobin (HbF) variability is a possible factor which could suppress HbS production causing variable responses to the RCE’s, however, generally HbF levels are at similarly low levels among patients with HbSS. We also considered variability in lifetime of the transfused RBCs and the storage age of the RBC units, but most of the transfused red cells should survive well beyond the 3-4 weeks between RCEs.
The time interval between post-HbS and F/u-HbS is one of the most significant confounders for the relationship. Some SCA patients have compliance issues with their chronic red cell exchange schedules. The monthly RCE schedules continually changed around patients’ work/study schedules. Thus the time intervals vary widely causing a greater variability in the F/u-HbS. Our cases were retrospective, but the time interval can be more uniform in a well - controlled prospective study.
The cut-off post-HbS
We were able to demonstrate more consistent outcomes in patients who were exchanged to HbS 5-10% by RCE. Though the sample size of patients is small (n=14), we found significant differences in F/u-HbS levels for different post-HbS level ranges. Using Fischer’s Exact test, we demonstrated that lowering the post-HbS 5-10% by using RCE was more effective than allowing it to exceed 10% (p=0.001). We were also able to show that there is no difference between lowering the post-HbS to 10-15% vs 15-20%.
Limited studies suggested that the risk of alloimmunization does not increase with the number increase of red cell units for RCE comparing the unit number used for simple transfusion.23 Nonetheless, limiting the number of red cell units used decreases the likelihood of infectious diseases through exposure to donor blood products. Our goal is to minimize the units of RBCs exchanged while maintaining HbS below 30% between exchanges to balance the risk of transfusion-associated infection and the amount of RBC units needed.
Since 2013, the evidence for the use and efficacy of RCE for stroke prophylaxis and iron overload prevention in SCA patients has increased. The therapeutic apheresis guidelines were modified in 2016 to account for this expanding evidence. Most importantly for our study, the indication of RCE for stroke prophylaxis and iron overload prevention in SCA patients has changed from a category II to I and grade 1C to 1A. The guidelines have maintained that RCE is indicated for acute stroke and is considered category I, grade 1C.14,15
Our recommendation
In this study of RCE events for HbSS patients over a 15-year period, we demonstrated a method for ensuring HbS remains below 30% between exchange procedures in most cases. We also demonstrated that reducing post-HbS to 30% is not sufficient to keep HbS consistently below 30% for a month. We recommend decreasing post-HbS to 5-10% in order to comply with this current treatment guideline for pediatric SCA patients of keeping HbS < 30% prior to the follow up monthly RCE.
While a large number of RCE events were studied (N=272), this study represents the experience of only 14 patients. This is a small number when considering biological variability including treatment responses. This small patient sample size could limit the generalizability of our findings in ways that are unknown.
A prospective randomized trial with a larger number of patients, especially in the category of post-HbS 5-10%, with adequate statistical power, and also with more standardized time interval between post-HbS and F/u-HbS, is warranted to provide more insight for endpoints including but not limited to F/u-HbS and co-morbidity improvement.
This study highlights the difficulty of maintaining low HbS in pediatric SCA patients, especially over many years. The cut-off post-HbS level will likely lie around 10% in order to maintain most of the patients’ F/u-HbS < 30% , which is in compliance of current pediatric SCA treatment guidelines. To our knowledge, this is the first study which demonstrates a possible approach to ensure HbS levels remain mostly < 30% between monthly RCE therapies.
The authors would like to acknowledge our apheresis nurses for performing all the red cell exchange procedures for sickle cell anemia patients, and drawing specimens for RCE related tests as needed. We thank the laboratory staff for performing all the laboratory tests in the study, and we thank the clinical staff for referring their patients to our care. We appreciate Statistician Dr Yungtai Lo’s help for the statistics analyses.
The authors declare that they have no competing interests.

©2018 Himchak, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.