Advances in
eISSN: 2573-2862


Review Article Volume 3 Issue 2
Department of Biology, University of Hawaii at Hilo, USA
Correspondence: Li Tao, Department of Biology, University of Hawaii at Hilo, 200 West Kawili Street, Hilo, Hawaii 96720, USA
Received: March 30, 2018 | Published: May 15, 2018
Citation: Martin LD, Tao L. “Braking Bad”: Halting Mitotic Motors in Cancer Cells. Adv Cytol Pathol. 2018;3(2):49-51. DOI: 10.15406/acp.2018.03.00051
Cancer continues to be a leading topic for research. The devastating effects of neoplastic cell transformation have been described for eons and there have been perpetual efforts to rid our species of this disease. Unregulated cell proliferation is the primary feature of tumors and research has focused on disrupting and halting the cell cycle in these tissues. During the last 50 years, post-translational or "epigenetic" modifications of transcribed proteins have been recognized to be of equal (if not greater) influence than DNA genetic mutation in the transformation to tumor cells. Mitosis represents a vulnerable point in the cell cycle to apply the brakes to unregulated tumor cell propagation. Specifically, the characterization of the complex regulation of mitotic motors, crucial for cytokinesis, is underway in numerous laboratories. In this article, we review the advent and current state of our understanding of epigenetic influence on neoplastic cell growth and potential targets to inhibit uncontrolled tumor cell division.
Keywords: cancer, cytokinesis, cell division, cell growth, microtubular motors, cells, apoptosis, cell death, cell division, microtubulin, kinesin
PTM, post-translational modification; HAT, histone acetyltransferase; HDAC, histone deacetylase; CENPE, centromere-associated protein E
Cancer has plagued mankind for eons. In fact, there are records of bone cancer in ancient texts and Egyptian mummies dating from 1600 B.C. Often causing severe pain, disability and death, there has been a singular focus on the causes and treatments of cancer over time. Simply put, this disease is characterized by the unregulated division/propagation of previously normal cells in a particular area of the body. Cancer is the second most common cause of death worldwide affecting approximately 50 percent of American men and one third of American women.1
The cost of caring for cancer patients in the United States is monumental. According to the American Cancer Society, insurers pay about $88 billion per year for cancer-related therapy. Approximately $4 billion is paid by the patients ("out of pocket").2 Though the incidence of cancer is declining and more patients are surviving the disease, much work remains in order to mitigate the devastating human and financial toll.
Apoptosis in disease development
The existence of a multicellular organism is predicated on a balance of continual cell proliferation and cell death. In the development of a vertebrate embryo, almost half of the nerve cells undergo cell death soon after formation. In adult tissues, cell division will closely approximate cell demise. Unlike the death of injured cells, a programmed cell death (termed apoptosis) does not damage surrounding cells with the inflammation created by trauma to cells. In fact, billions of cells die every hour in the adult intestine and bone marrow. This intricate balance is essential to the health of the entire organism. The loss of this balance can lead to a variety of disease processes in the affected organism. Indeed, the uncontrolled proliferation of cells may lead to neoplastic disease. As might be imagined, the focus of cancer therapy in the past 50 years has been to impede or halt the uncontrolled division of these abnormal cells.
Regulatory mechanisms of post-translational modifications
The post-translational modification (PTM) of proteins has been identified as a central regulatory mechanism in the intricate balance of all life. In the early 1960's, pioneering work by Dr. Vincent Allfrey and his colleagues hinted that the human proteome is vastly more intricate than the human genome. This work followed shortly after the first description of a PTM, protein phosphorylation, reported by Fischer et al.3 Protein phosphorylation was feverishly studied over the ensuing decades due to its regulation of numerous cellular functions, such as metabolic pathways and signal transduction.
Several years later, a second PTM, protein acetylation, was described by Phillips in 1963.4 At that time, Allfrey began to study the regulation of gene expression by chromatin. His work demonstrated that a radio labeled acetate (acetate-2-C14) was taken up and incorporated into histones and that this process was not sensitive to a translational inhibitor (puromycin).5 This work suggested that this process occurred after the translation of the polypeptide by mRNA. Though the focus of PTM research over the next 30 years was primarily on protein phosphorylation, Allfrey continued to work actively to further understand protein acetylation. Likely unknown to Dr. Allfrey at the time, his work laid the foundation for our refined understanding of the epigenetic regulation of gene expression. Initially, the term was used to describe the nebulous process by which a fertilized zygote develops into a mature organism.6 This was a largely imprecise and poorly understood concept until several groundbreaking studies in the last half of the last century changed the concept of epigenetic control of genetic expression. One of these studies involved purification of bovine thymus protein using an affinity matrix with trapaxin (a histone deacetylase inhibitor).7 Trapaxin had been described by Yoshida et al.,8 as a small molecule that could prompt tumor cell differentiation.8,9
Anti-mitotic targets in development of cancer therapy
Within the next 10 years, further elucidation of histone acetyltransferase (HAT) and histone deacetylase (HDAC) structures and the first proteomic screen for acetylation sites were accomplished.10 During this time, much of the clinical therapeutic approach to the treatment of various cancers focused on the refinement of a variety of anti-mitotic drugs to target spindle microtubules. The first of these drugs, taxanes, actually promote microtubule formation. Taxanes interfere, however, with microtubule disassembly, resulting in a cell clogged with microtubules and inevitable apoptosis. Another class of anti-mitotic therapy targeted the β-tubulin subunit of microtubules. These vinca alkaloids would therefore interfere with mitotic spindle formation and halt the cell cycle in metaphase. The interference with spindle function is largely effective in leading to mitotic arrest and apoptosis. Unfortunately, these therapies were associated with significant side effects such as immune suppression, bleeding, and peripheral neuropathy. There is also reported tolerance to these pharmaceuticals where the neoplasm may become resistant to initial beneficial effects. Clearly, a more refined therapy to combat neoplastic processes is needed. Many researchers have spent the past decade attempting to find a means to more specifically target and halt the uncontrolled propagation of cancer cells.
Current research into the role of mitotic motors in regulation of cancer cell division
There has been much interest in the role of mitotic motors in the regulation of cancer cell division. Kinesins, a family of motor proteins perform many intracellular functions including the formation of the mitotic spindle, as well as separation of the chromosomes during mitosis. In essence, kinesins have been strongly associated with oncogenesis in humans. As such, they represent a likely target for the development of alternative therapy to current anti-mitotic drugs. First discovered in the squid nervous system in 1985, subsequent work has led to the discovery of kinesin proteins in all eukaryotic cells.11 Forty distinct kinesin proteins have since been described in human cells. Kinesin motor proteins utilize hydrolysis of ATP to move along the microtubules. An enhanced understanding of the movement of kinesin proteins along the mitotic spindle may provide insight into new targets in the regulation of cancer cell division. What are the mechanisms by which kinesin motor proteins conduct their polarized movement along the microtubule tracks? Recent research has suggested that various post-translational modifications may significantly influence and regulate this process. Dr. Allfrey would likely be fascinated with the fact that post-translational modifications of microtubules indeed appear to regulate some important characteristics of cancer cell proliferation and migration. The kinesin family of proteins share a highly conserved motor domain, but perform a variety of distinct intracellular functions. There is strong evidence that post-translational modifications of the tubulin dimer in the mitotic spindle may “direct” the function of these mitotic motors in addition to other small ligands and molecules. Variations in the domains of tubulin, the result of genetic variation as well as post-translational modifications, appear to result in the functional heterogeneity of microtubules.12 Extensive post-translational modifications involve both the alpha and beta tubulin subunits. The best described of such modifications include polyglutamylation, detyrosination and acetylation. These modifications govern how various proteins bind and function on microtubules. Research by Reed et al.,13 demonstrated that the genetic elimination of alpha-tubulin acetylation at the Lys 40 site inhibits the binding and motility of kinesin- 1.13 Further studies suggest that post-translational modifications of tubulin are responsible for motor binding and velocity.14
Summary and proposed next steps
The regulation of active kinesin motors along mitotic spindles represents fertile ground for the exploitation of these proteins in the development of anti-tumor therapies. In the past few years, several molecules that appear to interfere with kinesin-5 and the centromere-associated protein E (CENPE) have begun clinical trials in the treatment of various neoplasms.15,16 Fascinating work with LaSOM 65 (a monastrol-derived compound) seems to lead to antiproliferative and proapoptotic consequences in glioblastoma cells in vitro. Previous neurotoxic effects prevalent with previous anti-mitotic chemotherapeutic agents are markedly diminished with this drug.14,17–19 Other actions of kinesin inhibitors have been increased antineoplastic effects in combination therapies as well as diminished angiogenesis by an adverse effect on the vascular endothelium. As promising as this work appears, it should be noted that the regulation of these motor proteins is quite complex and the full nature of the regulatory pathways have yet to be described completely. The Tao group has found that, contrary to our previous understanding, not all kinesins move along the microtubules independently. He described the need for the protein RacGAP to "activate" kinesin-6 in order to prompt its movement along the microtubular spindle.20 (Figure 1).
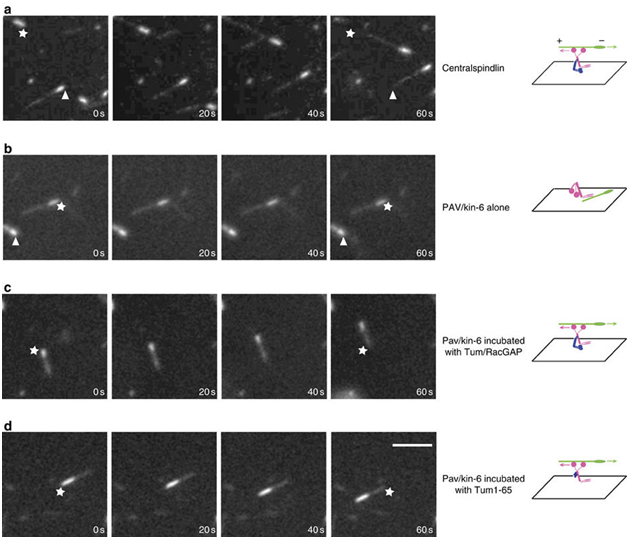
Figure 1 MT gliding assays reveal Tum/RacGAP is required for the motor activity of Pav/Kinesin-6. Assays of MT gliding driven by (A) centralspindlin, (B) Pav/Kinesin-6, (C) Pav/Kinesin-6 incubated in vitro with Tum/RacGAP, (D) Pav/Kinesin-6 incubated in vitro with Tum1-65 fragment. In each row of panels, stars and arrowheads are positioned in the same spot to judge movement. Polarity and direction of movement is indicated by a brighter minus-end. Scale bar 5 micrometers. Figure and description courtesy of Dr. Li Tao.
This may provide a yet more refined target to halt the cytokinesis and division of neoplastic cells. As may be obvious with the numerous varied intracellular roles of kinesins, the regulation of these proteins is complex and, as yet, poorly defined. The kinesins do have the advantage of much diminished neurotoxicity that has been associated with prior anti-mitotic agents such as taxanes and vinca alkaloids. Some of the studies of kinesin protein inhibitors have, however, been disappointing in that they failed to display the in vivo effects in humans as observed in xenografts. The trials of the Eg5 inhibitor ispinesib were flawed in that the human tumors displayed a much slower mitotic rate than the pre-clinical xenograft trials.21 Targeting kinesin motor proteins has also met with difficulty in the development of resistance of the neoplastic cells to the therapy. For example, mitotic arrest of tumor cells and apoptosis by kinesin inhibitors has been shown to increase the release of a heat shock protein, HsP70, that has antiapoptotic effects.22 Therefore, a combination of therapies including kinesin inhibitors may yield a more efficacious approach to the development of treatment for specific cancers. Recent studies have demonstrated improved efficacy in the treatment of cancer with a combination of kinesin inhibition and other therapies (such as augmentation of tumor suppressor genes). If other kinesins require "activation" by co-proteins (as Dr. Tao described with kinesin-6), they could, in combination with other modalities, provide a means to significantly retard or even abort the uncontrolled cell division pathognomonic of the neoplastic process. In any case, it is clear that "applying the brakes" to mitosis will likely require a larger view of kinesin regulation. Through improved understanding and application of kinesin regulation, in combination with other synergistic anticancer therapies, we will undoubtedly take a leap forward in our battle with this devastating disease.
NIH INBRE III Pilot Grant to L. Tao
Leah Martin and Li Tao declare that there is no conflict of interest.

©2018 Martin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.