Advances in
eISSN: 2573-2862


Research Article Volume 2 Issue 1
1Department of pathology, Albert Einstein College of Medicine, USA
2Yeshua University, USA
3Department of epidemiology and population health, Albert Einstein College of Medicine, USA
Correspondence: Yanhua Wang, Department of Pathology, Albert Einstein College of Medicine/Montefiore Medical Center, 111 East 210 Streets, Bronx, NY 10467, USA
Received: October 26, 2016 | Published: January 24, 2017
Citation: Wang Y, Khader S, Mehlman Y, et al. Loss of ?II and ?III spectrin isoforms in body fluids is associated with metastasis Adv Cytol Pathol. 2017;2(1):5–9. DOI: 10.15406/acp.2017.02.00007
Background
Spectrins are the principal skeletal proteins involved in cellular adhesion, migration, and signal transduction, and have been shown to be associated with integrate structure and function in complex tissue of all multi cellular organisms. The present study investigates the role of spectrins in metastasis.
Methods
Three categories of samples were studied. First, Spectrin expression patterns were examined in 66 normal, benign and malignant samples. Second, 188 primary tumor cases, including 69 counts of metastasis, were studied. Finally, 29 cases of body fluid metastasis without available primary tumor samples were studied. Expression of spectrin isoforms aII, bI, bII, and bIII were studied by immunohistochemistry in primary tumors (188 cases) with lymph node, remote organ, and body fluid metastasis; the 26 cases of malignant body fluids were paired with their corresponding primary tumors and analyzed.
Results
In this paper we focus on the expression of aII and bIII isoforms. We found an aberrant loss of aII mainly in breast invasive ductal carcinoma, among other cancers. Positive body fluids had markedly reduced aII and bIII expression, (P<0.0009, P<0.0001 respectively). bII remained present in all the primary tumors and cytology samples. No difference was identified in lymph node or remote organ metastasis.
Conclusion
We posit based on the established role of spectrins in various forms of cancer and in cell structure and adhesion that spectrins play an important role in maintaining tissue structure, with aberrant expression associated with body fluid metastasis.
Keywords: spectrin, carcinoma, cytology, metastasis, effusion
Spectrins are major structural proteins and are composed of a and b subunits. Together with the flexible actin-binding domains at the end of each subunit, spectrins are a part of the critical a-actin cell adhesion and signal transduction pathway. These a and b subunits are associated laterally to form anti parallel heterodimers, which are later assembled in a head-head structure to form heterotetramers, thereby acting as cyto skeletal support for the plasma membrane.1 Spectrins play an important role in the organogenesis of various different sites. Many of the physiological characteristics of normal epithelium, such as polarization, specialized differentiation, and adhesion and mechanical support, depend, at least in part, on the coiled structure of spectrins.2
Spectrins have been further related to cellular morphologic changes by their connection to phosphodiesterase and cAM P.3-4 It is believed that spectrins are also involved in signal transduction, thereby regulating normal cellular function, cellular proliferation, and cell cycles.5-8 . Importantly, spectrin loss has been shown to impair cell adhesion and cause cell spreading. These cell adhesion defects are associated with modification of the actin cytoskeleton, including loss of stress fibers, alteration of focal adhesions, and modified expression of some integrins.9 Spectrins have also been shown to have a role in breast and lung cancer.10-12 Hence, aberrant changes of spectrin expression are associated with tumorogensis, cell spreading, and metastasis.
In summation, spectrin isoforms are multifunctional molecules composed of a- and ß-subunits that tetramize, link ankyrin to the plasma membrane, and help integrate structure and function in complex tissues. The expression pattern of spectrin isoforms has not been comprehensively studied in benign, malignant, or metastatic epithelial tumors. However, due to the established role of spectrin in lung and breast cancer, as noted above, coupled with the role of spectrin in the general physiological characteristics of the cell, such as cell adhesion and support, we hypothesize that spectrin isoforms may be involved in the metastatic progression of malignant epithelial tumors. Therefore, in this study we seek to determine the distribution and localization of spectrin isoforms in a large number of primary carcinomas, regional lymph node metastases, and positive body fluid cytology samples using immunohistochemical staining of a large number of epithelial neoplasms.
Tissue samples
Three categories of samples were studied: 66 cases for initial review of spectrin expression patterns 188 primary tumor cases, including 69 counts of metastasis; and 29 cases of body fluid metastasis without available primary tumor samples were studied. The initial 66 normal, benign, and malignant epithelial neoplasm cases (107 expression counts) were analyzed for basic spectrin isoform expression, helping to identify how to proceed with further study of the cases of carcinoma and metastasis, as below.
Subsequently, a panel of 188 cases of primary carcinoma was selected for analysis. Cases included colon (N=77), stomach (N=3), breast (N=9), endometrium carcinoma (N=3), ovary carcinoma (N=3), lung carcinoma (N=91, adenocarcinoma N=76 and squamous cell carcinoma N=15), and thyroid (N=2). 69 counts of metastasis were included within this cohort of 188 carcinomas, as follows: regional positive lymph nodes (N=33) from colon and breast; remote organ metastasis (N=10) from colon, thyroid, and breast, to lung and liver; and malignant body fluids (N=26), primarily from the colon, stomach, liver, breast, uterine endometrium, ovary, and lung. These 26 cases of malignant body fluids were paired with their corresponding primary tumors and analyzed. Some of these 69 metastatic samples represented single patients with multiple forms of metastasis; thus, this count of 69 metastasis samples does not equal 69 individual cases, as one case can have more than one metastasis.
In addition to the 188 primary cases, there were 29 cases of positive body fluid without available corresponding primary samples, totaling 55 cases of positive body fluid (26 with available primary tumors and 29 without available primary tumors). Paraffin blocks were retrieved from the files of the Department of Pathology at Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY. The Institutional Review Board of Montefiore Medical Center, Bronx, NY granted us the permission to use clinical information and tissue samples for research purposes.
Tumor microarrays
For larger tissues, tumor microarrays were constructed from formalin-fixed, paraffin-embedded samples using a manual tissue arrayer (Chemicon International Temecula, CA). To ensure adequate sampling, each case was represented in triplicates using 1.0 mm cores. For smaller biopsy samples, histologic contiguous sections were individually prepared. The immunohistochemical staining conditions for spectrin isoforms aI, aII, ßI, ßII and ßIII are the same as previously published.13 A particular spectrin isoform was scored as positive if it was expressed by at least 20% of the cells.
The intracellular localization of each spectrin isoform was recorded as sub membranous, cytoplasmic granular, nuclear, or peri nuclear dot-like. Sections of paraffin-embedded tissue from reactive tonsils were used as a positive control tissue. In general, all spectrin isoforms were easy to score because of their distinct staining patterns.
Statistical analysis
Differences in aII spectrin expression and ßIII spectrin expression in the positive body fluid and primary tumor were compared using Mc Nemar’s tests. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Gary, NC). A P value less than 0.05 was considered statistically significant.
The expression of spectrin isoforms in normal and neoplastic epithelial tissues is summarized in Table 1. Expression of 55 cases of body fluid metastasis, composed of 26 cases with available primary tumors for paired study (as below) and 29 cases with no available primary tumors, is summarized in Table 2.
Tissue type |
Cases |
aII |
bI |
bII |
bIII |
Colon |
|||||
Normal |
3 |
+ |
- |
+ |
+ |
Tubular adenoma |
4 |
+ |
- |
+ |
+ |
Carcinoma |
3 |
+ |
- |
+ |
+ |
Liver |
+ |
+ |
|||
Normal |
3 |
+ |
+ |
+ |
+ |
Hepato cellular carcinoma |
2 |
+ |
- |
+ |
+ |
HCC*, glandular pattern |
1 |
+ |
- |
+ |
+ |
Bile ductules |
|||||
Normal ductule |
3 |
+ |
- |
+ |
+ |
Adenocarcinoma |
3 |
+ |
+ |
+ |
+ |
Kidney |
|||||
Normal |
4 |
+ |
- |
+ |
+ |
Clear cell carcinoma |
2 |
- |
+ |
+ |
+ |
Prostate |
|||||
Normal |
3 |
+ |
+ |
+ |
+ |
Pin** |
3 |
+ |
+ |
+ |
+ |
Adenocarcinoma |
3 |
+ |
+ |
+ |
+ |
Urinary bladder |
|||||
Normal |
3 |
+ |
+ |
+ |
+ |
High grade dysplasia |
1 |
+ |
- |
+ |
+ |
Squamous cell carcinoma |
3 |
+ |
- |
+ |
+ |
Breast |
|||||
Normal lobules |
8 |
+ |
+/- |
+ |
+ |
Normal ducts |
8 |
+ |
- |
+ |
+ |
Apocrine metaplasia |
2 |
+ |
-/+ |
+ |
+ |
DCIS*** |
3 |
+ |
-/+ |
+ |
+ |
Invasive carcinoma |
3 |
+ |
- |
+ |
+/- |
Uterine cervix |
|||||
Normal |
3 |
+ |
+ |
+ |
+ |
Low grade dysplasia |
4 |
+ |
+ |
+ |
+ |
Carcinoma |
3 |
+ |
- |
+ |
+ |
Thyroid |
|||||
Normal |
9 |
+ |
- |
+ |
+ |
Follicular adenoma |
5 |
+ |
- |
+ |
+ |
Papillary carcinoma |
3 |
- |
+ |
+ |
+ |
Lung |
|||||
Normal bronchial mucosa |
3 |
+ |
- |
+ |
+ |
Normal lung |
3 |
+ |
- |
Weakly + |
+ |
Adenocarcinoma |
3 |
+ |
+/- |
+ |
+ |
Table 1 Expression of spectrin isoform in normal and neoplastic tissues
*Hepato cellular carcinoma
**Prostatic intraepithelial neoplasia
***Ductal carcinoma in situ
Tissue type |
Case(55) |
aII(%) |
bI(%) |
bII(%) |
bIII(%) |
Primary colon cytology |
5 |
3/5(60) |
0/5(0) |
5/5(100) |
1/5(20) |
Primary breast cytology |
9 |
4/9(44) |
0/9(0) |
9/9(100) |
2/9(22) |
Primary lung cytology |
20 |
16/20(80) |
10/20(50) |
20/20(100) |
6/20(30) |
Primary stomach cytology |
7 |
3/7(43) |
0/7(0) |
7/7(100) |
2/7(29) |
Endometrium cytology |
8 |
2/8(25) |
0/8(0) |
8/8(100) |
2/8(25) |
Primary ovary cytology |
4 |
3/4(75) |
0/4(0) |
4/4(100) |
2/4(50) |
Biliary/pancreas cytology |
2 |
1/2(50) |
1/2(50) |
2/2(100) |
1/2(100) |
Table 2 Spectrin isoform expression in body fluid cytology cases
Marked reduction in the expression of spectrin isoform aII in body fluid malignant cells compared to the primary malignancy
aII spectrin was significantly reduced in positive malignant body fluid samples as compared to primary tumors in the paired study: The expression of aII spectrin in positive body fluid was 61.5% (16/26) versus 100% (26/26) of primary tumor (p<0.001) (Table 2 & Figure 1). Positive cytology cases with negative staining of aII spectrin tended to be single celled with high nuclear grade (Figure 3C), whereas tumor metastasis to lymph nodes and remote organs usually formed tumor nests and glandular structures (Figure 2B, 2C & 3B), as represented by colon and invasive ductal breast carcinoma. The cytoplasmic membrane stain of aII spectrin of malignant tumors did not appear in as sharp a linear formation as was seen in the benign epithelial cells.
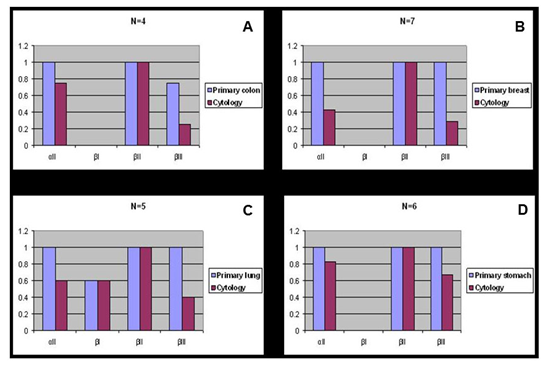
Figure 1 Spectrin isoform expression in paired surgical resection primary versus positive cytology cases.
A) Primary colon adenocarcinoma versus positive cytology.
B) Primary breast carcinoma versus positive cytology.
C) Primary lung carcinoma versus positive cytology.
D) Primary stomach carcinoma versus positive cytology.
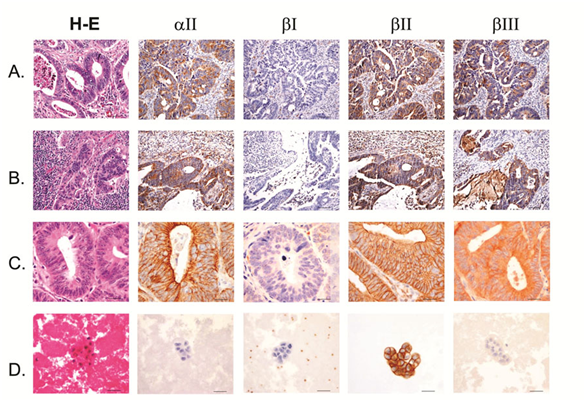
Figure 2 Colon adenocarcinoma with lymph node, remote organ and body cavity metastasis.
A) Primary colon adenocarcinoma.
B) Colon adenocarcinoma metastatic to lymph node.
C) Colon adenocarcinoma metastatic to lung.
D) Positive pleural fluid from colon adenocarcinoma. Scale bars, 40mm.
Significant aberrant loss of spectrin isoform ßIII in positive body fluid compared to the primary tumor
Similar to the aII spectrin, the ßIII spectrin was significantly less expressive in positive body fluid samples when compared to its primary counterpart. In general, ßIII spectrin was expressed in all normal tissue, and in benign and premalignant lesions. The majority of primary malignant tumors as well as tumors metastasized to the lymph nodes and remote organs also expressed ßIII. In such metastasized tumors to the lymph nodes and remote organs, the tumor cells usually had similar structures as their primary tumors (Figure 2B, 2C & 3 B). Paired studies revealed a significant aberrant loss of ßIII spectrin expression in the body fluid of the positive cytology cases as compared to the primary tumors; this positive rate was determined to be 38.5% (10/26) and 92.3% (24/26), respectively (P<0.001) (Table 3 & Figure 1, 2D & 3C).
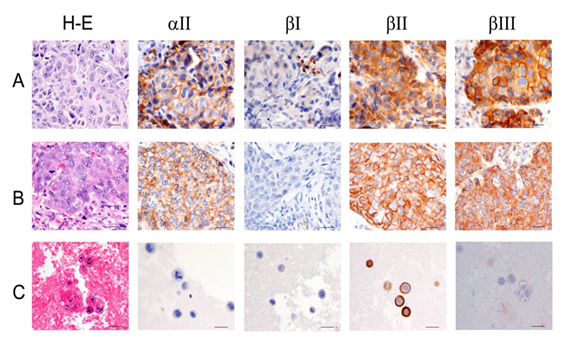
Figure 3 Breast carcinoma with lymph node and body cavity metastasis.
A) Primary breast carcinoma.<br />
B) Breast carcinoma metastatic to lymph node.<br />
C) Breast carcinoma with positive body fluid. Scale bars, 40mm.
The staining pattern of ßIII spectrin in normal epitheliae and benign lesions showed fine cytoplasmic granules, while in the cytoplasm of malignant neoplasms the staining appeared as disorganized coarse or clumped granular staining; this is markedly different than other forms of spectrin, which showed a linear cytoplasmic membrane stain. The staining intensity of spectrin isoform ßIII in cytology samples also tended to be weaker when compared to its solid counterpart.
Universal expression of ßII spectrin in malignant primary and metastatic malignant tumor
ßII spectrin was universally expressed in normal, benign, and malignant tumor cells. As shown in Tables 1-3 & Figures 2-3, no difference was noted between the primary tumor and its corresponding secondary positive body fluid sample. Comparison between the primary tumor and metastasis to lymph nodes and other organs showed no difference in the expression of ßII spectrin (Figures 2-3). However, ßII spectrin isoform stained sharply linear under the cytoplasmic membrane in normal epithelae and benign lesions, while in malignant tumors ßII spectrin stained broader sub membranously and showed a more granular cytoplasmic distribution.
Carcinoma type |
No cases |
aII |
bI |
bII |
bIII |
Colon |
|||||
Primary tumor |
4 |
4/4(100) |
0/4(0) |
4/4(100) |
3/4 |
Body fluid |
4 |
3/4(75) |
0/4(0) |
4/4(100) |
1/4(25) |
Breast |
|||||
Primary tumor |
7 |
7/7(100) |
0/7(0) |
7/7(100) |
7/7(100) |
Body fluid |
7 |
3/7(43) |
0/7(0) |
7/7(100) |
2/7(29) |
Lung |
|||||
Primary tumor |
5 |
5/5(100) |
3/5(60) |
5/5(100) |
5/5(100) |
Body fluid |
5 |
3/5(60) |
3/5(60) |
5/5(100) |
2/5(40) |
Stomach |
|||||
Primary tumor |
6 |
6/6(100) |
0/6(0) |
6/6(100) |
6/6(100) |
Body fluid |
6 |
5/6(83) |
0/6(0) |
6/6 (100) |
4/6(67) |
Endometrium |
|||||
Primary tumor |
2 |
2/2(100) |
0/2(0) |
2/2(100) |
2/2(100) |
Body fluid |
2 |
1/2(50) |
0/2(0) |
2/2(100) |
1/2(50) |
Ovary |
|||||
Primary tumor |
1 |
1/1(100) |
0/1(0) |
1/1(100) |
1/1(100) |
Body fluid |
1 |
0/1(0) |
0/1(0) |
1/1(100) |
0/1(0) |
Biliary/ Pancreas |
|||||
Primary tumor |
1 |
1/1(100) |
1/1(100) |
1/1(100) |
0/1(0) |
Body fluid |
1 |
1/1(100) |
1/1(100) |
1/1(100) |
0/1(0) |
Table 3 Spectrin isoform expression in paired primary tumor and positive body fluid sample)
Our results confirm the presence of aII, bII, and bIII spectrins in normal, benign epithelae, and endothelial cells as part of each cell’s normal histologic structure and function. The spectrin isoforms presented as sharp linear sub membranous or fine cytoplasmic granules. Several changes were noted in the presentation of spectrin isoforms in malignant neoplasms: Isoforms aII and bII lost their sharp linear sub membranous staining; bIII appeared as coarser, clumped granules when compared to normal or benign conditions, indicating polarity loss in malignancy.
In addition to a qualitatively distinct presentation of spectrin isoforms in malignant neoplasms, an aberrant loss in quantitative expression of some isoforms was noted in malignant neoplasms with tendencies to metastasize to body cavities: This advanced clinical stage showed a loss of spectrin isoforms aII and bIII. The correlation between the aberrant loss of spectrin isoform expression in an advanced clinical stage of metastasis supports the observations of other in vitro studies mentioned above that posited the role of spectrins in cellular adhesion and in morphologic integrity and proliferation. The correlation between an advanced metastatic stage, one which notably defies several cellular adhesion and proliferation principles, and a loss of expression of a protein such as spectrin is quite logical and provides a solid basis for our findings.
We also report the finding that isoform ßII is universally expressed by normal, benign, and malignant epithelia cases studied. Only a slightly broader staining of isoform ßII under the cytoplasmic membrane was seen in malignant carcinoma, indicating the role of spectrin in maintaining the morphology of the cell.
The aberrant gain or loss of spectrin in malignant neoplasms is associated with a worse clinical outcome; this is especially true of the marked loss of aII and/or ßIII in malignant body fluids that predict an advanced clinical stage. These findings can be used to further implicate spectrin in key cellular functions related to metastasis, such as cellular proliferation and adhesion, corroborating previously published findings. This study represents only a window into the world of spectrin expression, with a more detailed molecular study composed of a larger cohort of body fluid samples needed to elucidate the details of their role, along with further studies needed to determine the precise mechanism by which spectrin influences neoplasms.

©2017 Wang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.