eISSN: 2378-3176


Research Article Volume 1 Issue 1
1Population Science Program, Center for Clinical Research, Southern Illinois University School of Medicine, USA
2Department of Medical Microbiology, Immunology and Cell Biology, Southern Illinois University School of Medicine, USA
3Simmons Cancer Institute, Southern Illinois University School of Medicine, USA
4Department of Surgery, Division of Urology, Southern Illinois University School of Medicine, USA
Correspondence: Wiley D. Jenkins, Population Science Program, Center for Clinical Research, Southern Illinois University School of Medicine, 801 North Rutledge, Springfield, Illinois, USA, Tel 217-545-8717, Fax 217-545-0799
Received: September 10, 2014 | Published: October 20, 2014
Citation: LeVault K, Mueller G, Wilber A, et al. Within-state geographical variations in renal cell carcinoma incidence: rural disparities in the state of Illinois. Urol Nephrol Open Access J. 2014;1(1):15-20. DOI: 10.15406/unoaj.2014.01.00004
Objectives: Rural-urban disparities in renal cell carcinoma (RCC) mortality have been observed, and we sought to determine if increased male RCC incidence rates in six contiguous counties (S6Co) in rural southern Illinois (IL) could be explained by known risk factors or require consideration of other etiological influences.
Methods: We analyzed incidence and behavioral risk factor data for the S6Co, similarly rural IL and US counties (rural-urban classification codes 7 through 9; ILRUCC 7-9 and USRUCC 7-9), the remaining IL counties (ILr), and total IL and US.
Results: ILRCC rates are higher than the US at both the total population (22.2/100,000 [95% CI=21.8-22.6] vs. 19.9 [19.8-20.1]) and rural (RUCC7-9; 23.7 [21.7-25.8] vs. 20.7 [19.9-21.5]) levels, with S6Co the highest (30.8 [24.8-37.5]). Within IL, the S6Co RCC rate remained significantly increased compared to both ILr and ILRUCC 7-9 after adjustment for smoking, obesity and hypertension.
Conclusion: Rural IL males experience increased RCC risk which may be attributed to a locally-specific combination of genetic, environmental and cultural factors. State-level chronic disease control programs should examine how such factors may significantly vary across relatively small geographical scales.
Keywords: renal cell carcinoma;, rural disparities, heritable risk factors
RCC, renal cell carcinoma; S6Co, six contiguous counties; IL, illinois; SEER, surveillance, epidemiology and end results; IDPH, illinois state cancer registry; IL-BRFSS, illinois behavioral risk factor surveillance system; RUCC, rural urban continuum codes; CI, confidence intervals; IRRs, incident rate ratios
Kidney cancer, comprising renal cell and renal pelvis carcinomas, is an important health issue with national incidence rates increasing an average of 1.6% each year over the last decade (from 12.9 to 15.3/100,000; 2002-2011).1 In 2014, kidney cancers were responsible for 63,920 new cases and 13,860 deaths.2 Renal cell carcinoma (RCC) is particularly concerning as it has an incidence nearly ten times that of renal pelvis, has an increasing incidence rate compared to a more stable rate for renal pelvis (RCC itself increasing 2.9% 2000-2009), and the majority of cases are diagnosed before symptoms develop due to improved abdominal imaging.3,4 However, despite the ability to detect smaller tumors, the rate of RCC-related deaths has not declined as 40% of patients present with metastatic disease and standard treatments (chemotherapy and radiation) are ineffective at that stage.5,6 As is the case with most diseases, prevention is the most effective treatment. For RCC, most risk factors are identifiable and amenable to effective intervention.7 There is therefore a need to identify the populations with increased RCC risk, and risk factors which they may be subject to, such that appropriate and sufficient resources may be prioritized.
Interventions are frequently directed towards those groups with increased risk of incidence or adverse outcomes (disparities). RCC has disparately impacted males (2006-2010 incidence rate of 21.0 vs. 10.6/100,000 females) and blacks (17.4 vs. 15.8/100,000 whites).3,7–9 However, little is known regarding the risk of RCC for the approximately 17-21% of the US population residing in rural areas.10 The fact that rural life occurs within a context of health and disease substantially different from more urban areas has led to the specification of rural populations as a priority population (also including racial and ethnic minorities, the elderly, etc.) for the Agency for Healthcare Research and Quality in 1999.11 Still, few publications describe possible rural/urban disparities in RCC incidence. One US study examined differences in urbanization at the state level and found that RCC mortality rates increase with rurality.12
There are multiple a priori factors suggesting that rural and urban differences in RCC do exist and may be significant. Etiologic disparities exist for risk factors disproportionately found in rural areas, such as increased rates of smoking and obesity and increased likelihood of hypertension and diabetes.13–16 Occupational and environmental exposures to carcinogenic compounds are inconsistently associated, though agricultural crop production and dry cleaning industries were identified as risk factors associated with clear cell RCC.17,18 Genetic factors influence an individual’s risk for developing kidney cancer, with the majority of hereditary RCC disorders being autosomal dominant with penetrance to multiple generations within affected families.19,20 It is entirely possible that there may exist a lesser degree of population genetic admixture in some relatively isolated rural populations (as has been demonstrated elsewhere)21 which may contribute to a relative concentration of heritable genetic risk factors.
Illinois (IL) is the 5th most populous state in the US and, with the exception of Chicago and other major metropolitan areas, is heavily farmed and less densely populated with significant proportions of county residents living in rural areas. As part of our Institution’s ongoing work in cancer epidemiology, prevention, and control in IL, preliminary analysis identified an area of six contiguous counties in southern IL (S6Co) which exhibited RCC rates disproportionate to IL as a whole and other rural IL counties. Residents of these counties may represent a more genetically homogeneous population as: there are only 31 people per square mile; 92% are white, non-Hispanic (except Pulaski County); and the majority report German, Irish, and English ancestry.22 Furthermore, there has been a large net migration out of southern IL due to declines in river trade, coal mining and increased incidence of flooding in the area over the past few decades.23
The objective of this study was to examine state and national surveillance data to determine if the significantly increased rate of RCC among males in the S6Co in rural Illinois area could be explained by risk factor adjustment or if other etiological factors need to be considered. While such data collection and analysis as presented here is specific to the population of Illinois and its cancer control program, the rationale and methodology are directly amenable to other state programs and chronic diseases. Such methods are also important for the elucidation of rural disparities for disease risk which have been infrequently studied.
Data sources
Epidemiologic data from Surveillance, Epidemiology and End Results (SEER) were compared with data from the Illinois State Cancer Registry (IDPH) for the 10-year period 2001-2010.24,25 County-level estimations of the rates of smoking, obesity and hypertension were based on data from the Illinois Behavioral Risk Factor Surveillance System (IL-BRFSS).26
Rural urban continuum codes (RUCC)
RUCC is a national classification scheme used to distinguish metropolitan counties by the population size of their metropolitan area, and non-metropolitan counties by degree of urbanization and adjacency to a metropolitan area.27 Nationally, these categories have been subdivided into three metropolitan and six non-metropolitan units. Based on these criteria, every county in a state is assigned one of nine codes (where 1=most metropolitan and 9=most rural).
Study design and variables
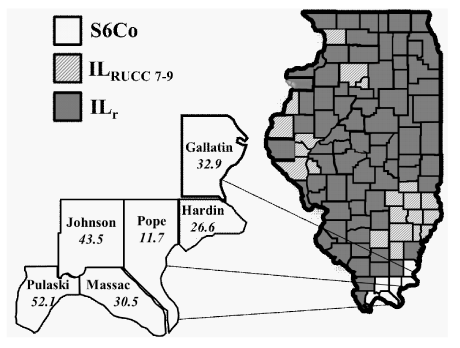
RCC data examined included: variation of incidence by degree of rurality (RUCC) and year of diagnosis. Illinois’ 102 counties were stratified by their 2013RUCC classification and separated into three groups: (i) The S6Co (described above) includes Gallatin, Hardin, Pope, Johnson, Massac, and Pulaski counties (nS6Co=6); (ii) ILRUCC 7-9 includes all other IL counties with a RUCC classification of 7-9 (similar rurality to S6Co; nIL-RUCC 7-9=21); and (iii) ILr is comprised of the remaining counties (nIL-r=75), as shown in Figure 1.
Statistical analysis
Statistical Analysis System software (SAS version 9.3) was used to calculate age-adjusted RCC incidence rates for IL and specific regions of interest (using 8 age groups defined by Illinois Department of Public Health [IDPH] State Cancer Registry’s publicly available data set) and all further data analyses. County-level behavioral risk factors (smoking, obesity, hypertension) were obtained from the IDPH Behavioral Risk Factor Surveillance System (IL-BRFSS). Descriptive statistics (e.g. mean; confidence intervals) and significant differences between values were determined by unpaired Student’s t-test (two-tailed) or one-way ANOVA with Tukey-Kramer’s post-hoc test with a Bonferroni correction. Poisson and negative binomial regression were used to model age-adjusted incidence rates for each gender by area and behavioral risk factor. These factors were modeled individually versus collectively due to regional sample size constraints. Negative binomial regression was used when over dispersion was present. Incident rate ratios (IRRs) were calculated and reported with 95% confidence intervals (CI) for each model. P-values are indicated with level of significance reported.
RCC incidence rates are increased for Illinois males
Illinois males experience increased RCC incidence compared to the US (22.2/100,000 [95% CI=21.8-22.6] vs. 19.9 [19.8-20.1]), and this increase is conserved when comparisons are restricted to include only more rural counties (ILRUCC7-9 23.7 [21.7-25.8] vs. USRUCC 7-9 20.7 [19.9-21.5]), and with S6Co the highest at 30.8 [24.8-37.5]. Overall, the incidence among S6Co males is approximately 50% greater than the US. For females we observed an analogous increase in incidence when comparing IL to the US, but most other comparisons have overlapping confidence intervals, and the incidence among S6Co females is only approximately 10% greater than the US.
Risk factor adjustment does not explain increased RCC incidence among rural Illinois males
We next examined county-level estimations of smoking, obesity and hypertension (Table 1).Comparisons were made for ILr, ILRUCC 7-9, and the S6Co.Among males, smoking varied across IL geographical groups with prevalence increased within the S6Co compared to ILr (31.5% vs. 23.6%) and ILRUCC 7-9 (at 22.5%). Alternatively, obesity did not significantly vary across the three groups, and hypertension showed modest but significant variability with ILRUCC 7-9 reporting greater prevalence than ILr(31.3% vs. 29.6%) but the S6Co was no different than either group (at 33.8%). Females demonstrated similar variations, with the addition that obesity and hypertension did trend significantly higher with increasing rurality (from 25.7% to 26.0% to 32.0% for obesity and 29.7% to 33.3% to 37.4% for hypertension; forILr, ILRUCC 7-9, and S6Co, respectively). We then calculated incident rate ratios while adjusting for each risk factor (Table 2). Here, we found that the RCC rate for S6Co males remained significantly increased compared to both ILr and ILRUCC 7-9, for each factor. No significant rate increases were observed for females. Due to the small sample size, it was statistically inappropriate to combine these factors into a single model.
These data present several interesting insights into RCC incidence in IL which may direct research and state-specific intervention efforts. First, we find that IL residents (male and female) experience an approximately 10% greater incidence of RCC. However, gender differences become apparent when examining more rural populations, with no significant difference observed for females between USRUCC 7-9 and ILRUCC 7-9 while the difference for males is significant with IL males more at risk than the US. Further, while males in the S6Co experience approximately 50% higher incidence than US males, females in this area suffer no such excess risk. For males the data were again striking as the incidence rate ratio for the S6Co was significant compared to ILr and ILRUCC 7-9 when adjusted for smoking, obesity, and hypertension? For females, no significant differences were retained. These data suggest that there is at least one etiologic factor which males in the S6Co disproportionately experience but for which we have not accounted.
Males in the S6Co experience a greater prevalence of smoking that those in other Illinois counties. However, adjustment for this disparity does not explain the increased RCC rate observed, and the prevalence of two other major RCC risk factors (obesity and hypertension) does not significantly vary. Still, we cannot assume that these factors do not have a significant contribution to RCC as some inherent weaknesses in how these county-level data are collected need to be considered. Such data are collected by random dialing of county residents. This data collection method may not achieve an adequate number of respondents in rural counties compared to more urban areas and with that a poorly representative data set. Furthermore, hypertension is reported as a physician’s diagnosis and no data is available regarding the degree and extent of medical control across the county. So while the hypertensive rate in the S6Co does not differ from other counties, we cannot speak to the levels of adequate control. For these reasons, and the overall decreased access to care present in the southern Illinois area, population-level interventions into the reduction of smoking and obesity, and an increase in the recognition and control of hypertension, would likely be quite beneficial in the S6Co area to address multiple health issues.
Enhanced RCC incidence rates for S6Co males may be attributed, at least in part, to factors which have received little study in rural America or IL: environmental exposures and altered genetic susceptibility due to isolated populations. Central and southern IL is heavily farmed, and farmers in particular have been shown to have excess risk of RCC in similar US populations.18 This increased risk of cancer was suggested to be related to environmental exposures. However, our limited understanding of the mode of action of many of these exposures, their complex effects on metabolic pathways and the possibility of co-exposure to other risk factors have made it difficult to establish any causal relationship to date. Specific to our study area, the S6Co population is disproportionately rural, with five counties having a 100% rural population and the remaining (Massac) at 51% rural. The S6Co thus represents 41.7% of all Illinois counties with a 100% rural population and 35.7% of counties with a rural population exceeding 80%. However, increased rurality is not necessarily equivalent to increased farming and exposure to agricultural chemicals (e.g. herbicides and pesticides). An examination of 2013 Illinois County Statistics from the US Department of Agriculture show that the S6Co counties are not near the low nor high ends of acreage planted for the Southwest and Southeast districts (comprising 24 counties) for four crops for which there is consistent data (wheat, corn, soybean, other hay; data not shown).28 Other potential sources of exposure, especially those which may be distributed across a substantial portion of the S6Co, are unknown.
Alternatively, isolated populations may experience an increase in disease risk effect size due to limited diversity of the population’s founding families and subsequent genetic drift. This may cause an increased frequency and attributable risk of specific disease susceptibility alleles, especially those that have a dominant or recessive pattern of inheritance.29 Furthermore, isolated populations share environmental and cultural factors. When combined with limited genetic diversity and increased rates of endogamy or decreased admixture, this can result in observable phenotypic variations. For example, isolated Sardinian villages sharing a common ancestry also display considerable phenotypic diversity.30 More recently, the Brazil municipality of Candido Godoi, founded by Polish and German immigrants in the late 19th/early 20th centuries, has an increased rate of twin birth attributed to genetic founder effect (loss of genetic diversity when a small number of individuals establish a new population).31 These data are pertinent to the S6Co as these communities have been established since the 18th century, largely of European immigrants of German, English and Irish ancestry.32 The limitation of travel before the adoption of the automobile would have contributed to limited population admixture and increased endogamy. Furthermore, the S6Co (and many other rural areas) have seen net out-migrations, further contributing to the concept of an isolated population subject to circumstances for founder effect observance.23Other factors may also have specific relevance to the increased RCC rate in S6Co males, as it has been shown that genetic variations and selections may differ by gender and that male germ lines may mutate at a greater rate than female.33,34
We acknowledge that there are limitations with this study. We used available data which is aggregated to the county level and cannot therefore assess individual levels of risk, behavior or cancer. Furthermore, we have no longitudinal data and can only make associations between current and concurrent rates of risk factors and cancer. While such factors and rates do not substantially vary over more extended time periods, we again relied upon aggregate data. On the other hand, these data are generally supportive of our a priori hypotheses, and support the plausibility of our etiologic arguments. Finally, the S6Co area borders five counties in northern Kentucky. While our concern was with RCC incidence in IL, it is not unreasonable to suppose that etiologic factors prominent in KY may exert influence in such bordering areas. However, this does not lessen the importance of identifying areas of increased risk within a state. In fact, findings like these can be overlooked if all rural counties are considered to have homogeneous risk.
This study presents novel findings regarding the epidemiology of RCC and how rural-urban disparities may be examined. While we focused on rural IL communities, our findings have general implications for further research as well as statewide cancer and other chronic disease prevention and control policies. There is clearly a combination of factors influencing the increased RCC risk among males in these six southern IL counties beyond that experienced by males in IL or the US that are similarly rural. Many of these rural populations tend to be relatively isolated, and there may be a specific combination of genetic, environmental and cultural factors causing an increase in cancer risk. However, there are no data concerning the actual geographical and genotypic isolation of rural communities, though variations in phenotype suggest they may occur. The identification of such populations has important implications and may be ideal for identification of known and novel heritable genetic signatures.29 Furthermore, many policy decisions regarding cancer screening, prevention and control are based upon the premise of equitable risk across populations, but our findings suggest this may be less accurate for more rural communities and especially when considered within the context of a state-specific program or plan.
None.
The author declares no conflict of interest.

©2014 LeVault, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.