eISSN: 2378-3176


Clinical Paper Volume 6 Issue 5
1Proffessor in urology in Al-Mustasyria College of medicine, Iraq
2Lecturer in AL Fallujah collage of medicine, Iraq
Correspondence: Omar M Shakir, Lecturer in AL Fallujahcollage of medicine & urosurgeon in AL Fallujah teachinghospital, Al-anbar, Iraq, Tel 00 9647905574549
Received: March 21, 2018 | Published: December 28, 2018
Citation: Azzawi ISA, Shakir OM. The efficacy & safety of trospium chloride in combination with tamsulosin for patients with lower urinary tract symptoms related to benign prostatic hyperplasia. Urol Nephrol Open Access J. 2018;6(5):182-187. DOI: 10.15406/unoaj.2018.06.00230
Background: While -blockers are recognized to be effective in management of LUTS associated with BPH, the role of antimuscarinic agents still needs to be addressed for the treatment of bladder over activity related to BPH. Our aim was to evaluate the efficacy, safety and tolerability of using a combination of Tamsulosin &Trospium chloride for men with LUTS related to BPH.
Methods: Prospective, controlled, clinical trial, included 71 symptomatic patients presenting with lower urinary tract symptoms secondary to benign prostatic hyperplasia (BPH). Patients were randomly divided into two groups, group 1 (n=36) treated with tamsulosin & trospium chloride and group 2 (n=35) treated with tamsulosin only. International Prostate Symptom Score (IPSS), with its quality of life score, post-void residual volume (PVR) and maximum flow rate (Qmax) was evaluated & they were followed for 2weeks of treatment.
Results: The mean age in group 1 was 61.9±7.97years (range 50 to73 years) while in group 2 it was63.1±7.43years (range 56 to73), the score of all the 3 irritative symptoms, dropped down in both groups, but the mean change was only significant for nocturia, and in favor of group 1. the significant difference in the mean change in obstructive symptoms collectively, was in favor of group 2 , while changes in the objective parameters of obstruction; PVR and Q max , were not significant between the 2 groups. The IPSSQoL score was significantly decrease in group 1, in comparison with group 2, which mean a better QoL in the group treated with Trospium. In both group there was a significant change in the IPSS from baseline but no statistically significant difference in the mean of change between the 2 groups.
Conclusions: Trospium chloride proved to be effective in controlling storage symptoms especially nocturia , which had a significant impact in improving QoL. Trospium chloride proved to be safe when used for BPH patients, as there was no retention of urine and no significant adverse changes in PVR and Q max.
Keywords: benign prostatic hyperplasia, overactive bladder, trospium chloride, tamsulosin
In BPH, the clinical symptoms of bladder outlet obstruction (BOO)are most likely due to combination of dynamic component mediated by prostatic smooth muscle contraction due to stimulation of Alpha1adrenoceptor static component mediated by mass related increase in urethral resistance).1
Literature showing that above fifty years of life, 25% of men suffer from lower urinary tract symptom (LUTS) that include voiding & storage symptoms , after age of 75years, the percentage become 50% ,in addition to that storage symptoms usually occur in 50-70%of patients with BPH ,They are more bothersome & affect quality of life (QoL) more than voiding symptoms, especially if they are associated with nocturia or incontinence.2−6
Many symptoms in men with BPH are related to obstruction induced changes in bladder function rather than to out flow obstruction directly.7
The causes of bladder over activity in men with BPH are not fully understood , and may be multi factorial , many pathophysiological mechanisms were postulated that initial response of detrusor muscle to obstruction is the development of smooth muscle hypertrophy & prolonged increase in vesical pressure during urination causing ischemia & leading to ischemic damage to neurons within the bladder (i.e denervation). Also there is evidence that obstruction may change neural-detrusal response that may lead to decrease bladder contractility, impaired central processing &altered sensation.1,8
Many researchers also found an increase in urinary level of nerve growth factor((NGF))* in patients with BOO with storage symptoms, which will decrease after successful medical treatment, and with obstruction , residual urine will increase &this will decrease the functional capacity of bladder &lead to frequency.9
Current medical treatment for BPH include ( 1adrenoceptor antagonists, 5 –reductase inhibitors, Phytotherapy & recently Phosphodiesterase 5 inhibitors).10-13
Although voiding symptoms are usually alleviated by the use of medicines (alpha1 blockers, 5 alpha-reductase inhibitors) or by TURP, storage (irritative) symptoms continue in 30-65% of patients.2
A significant number of patients with storage symptoms, that affect their quality of life, are in need to be treated with drugs that are capable of controlling their detrusor overactivity, Antimuscarnic drugs may be suitable in this aspect.14-17
In human bladder, all muscarinc receptors (M1-M5) are found. But there is a predominance of M2&M3 receptors in detrusor muscles, with M2 receptor predominate in at least 3:1 over M3 receptor, but there is a believe that M3 is more important in contraction.17
Anti muscarinic drugs are usually competitive antagonist &act during the storage phase to decrease urgency, frequency & increasing bladder capacity.17
Our objective is to evaluate the efficacy, safety and tolerability of using a combination of Tamsulosin ( 1 blocker) & Trospium chloride (anticholinergic agent) for men with LUTS related to BPH.
In this prospective clinical trial which was conducted from July 2015 to December 2016, 71 patients (50-73 years), presented to the our urology clinic (in outpatient clinic Yarmouk teaching hospital in Baghdad-Iraq ) who suffering from lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia(BPH) were consequently included. This study was approved by the ethical committee of our hospital.
Aim of the study was explained to the participant & verbal consent was obtained from them.
Randomization occurs by using two papers, one written on it 1 & other 2 & participant choice the paper randomly.
Assessment of patients was done via questionnaires that include sociodemographic variable such as name, age education, residency &contact information (mobile phone number).medical history such as chronic disease (D.M,HT…etc), a detailed history with implementation of IPSS (International prostatic symptom score) which is an 8 question (7 symptom questions +1 quality of life question) written screening tool used to screen for, rapidly diagnose, track the symptoms of and suggest management of the symptoms of the disease benign prostatic hyperplasia (BPH). Created in 1992 by the American Urological Association, it originally lacked the 8th QOL question, hence its original name: the American Urological Association symptom score (AUA-7) (See Table 1).18
In the past month |
Not at all |
Less than 1 in 5 times |
Less than half the time |
About half the time |
More than half the time |
Almost always |
Your score |
||
Incomplete Emptying |
0 |
1 |
2 |
3 |
4 |
5 |
|||
Frequency |
0 |
1 |
2 |
3 |
4 |
5 |
|||
Intermittency |
0 |
1 |
2 |
3 |
4 |
5 |
|||
Urgency |
0 |
1 |
2 |
3 |
4 |
5 |
|||
Weak stream |
0 |
1 |
2 |
3 |
4 |
5 |
|||
Straining |
0 |
1 |
2 |
3 |
4 |
5 |
|||
Nocturia |
0 |
1 |
2 |
3 |
4 |
5 |
|
||
Total I-PPS score |
|||||||||
Score: 1-7: mild 8-9: moderate 20-35: severe |
|||||||||
Quality of life due to urinary symptoms |
Delighted |
Pleased |
Mostly satisfied |
Mixed |
Mostly Dissatisfied |
Unhappy |
Terrible |
||
If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that? |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
||
Table 1 International prostate Symptom score
Physical examination (including DRE & Brief neurological examination) and investigations that include Lab. Investigations included Urinalysis, Blood urea, serum creatinine and Serum PSA. While imaging included Abdominal U/S (with concentration on prostate size & post voiding residue (PVR) and Uroflowmetry; to detect maximum flow rate (Qmax).
Inclusion criteria
Exclusion criteria
The participant randomly by ------ allocated in to two groups
Group 1(therapeutic)
This group treated with tamsulosin capsule 0.4mg once daily plus trospium chloride tablet 20 mg twice daily 1hour before meals.
Group 2(controlled)
This group treated with tamsulosin capsule alone.
At the end of 2weeks of treatment, therapeutic effect was assessed by re-evaluation of patients using
Data entry was done with S.P.O.S version, qs used & t test for analysis of variables. Student’s t test for comparison of means (quantitativ data) & the chi-square test for the comparison of percentages (qualitative data). P. value considered significant when it is equal to or less than 0.05.
In this prospective controlled study, 71 patients with moderate to severe LUTS were included. They were 36 in group 1 and 35 in group 2.
The mean age in group 1 was 61.9±7.97 years (range 50 to73 years) while in group 2 it was 63.1±7.43years (range 56 to73) without a statistically significant difference (P=0.792) as in Figure 1.
The mean value of prostatic size in group 1 was 37.1±10.19 while in group 2 it was 33±10.6 without a statistically significant difference (P=0.285) as in Figure 1.
After 2 weeks of treatment, patients in group 1&2 had significantly lower IPSS from baseline; in group 1 the mean of change - 8.3±2.61,while in group 2 the mean of change was -8.2±3.63 &no statistically significant difference was observed between them (P=0.909) as in Figure 2.
Quality of life score was also improved significantly from baseline in both groups. Compared with the group 2, (mean of change -1.2±0.76), significant change in QoL subscore was demonstrated in group1 (mean of change -2.05±0.94), (p =0.018) as in Figure 2.
Changes in obstructive symptom score (incomplete emptying, intermittency, weak stream, straining) were: in group 1, the mean of change - 3.7±2.02 while in group 2 the mean of change -5.4±1.53 with statistically significant difference was observed (P=0.011) as in Figure 3.
Changes in maximum flow rate were: in group 2 mean of change was +2.8±3.35 while in group 1, mean of change was +2.28±1.75, with statistically no significant difference was observed (P=0.810) as in Figure 3.
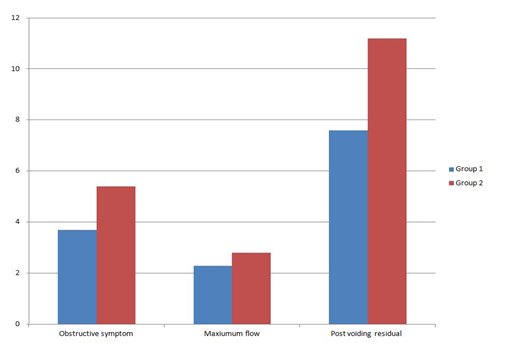
There was no significant difference in post voiding residual volume between the 2 groups (P = 0.266). Mean of change in group 1 -7.6±11, while mean of change in group 2 -11.2±7.9 as in Figure 3.
Urgency subscore was reduced significantly from baseline in both groups. The group 2 mean of change - 0.6±0.50. More reduction in IPSS urgency subscore was demonstrated in the group1 with a mean of change -1.15±1.18, but it was statistically non significant difference (P=0.094) as in Figure 4.
Nocturia subscore was also reduced significantly from baseline in both groups. Compared with the group 2 (mean of change - 1.2±0.95), significant reduction in IPSS nocturia subscore was demonstrated in group 1(mean of change - 2.3±0.73), (p=0.04) as in Figure 4.
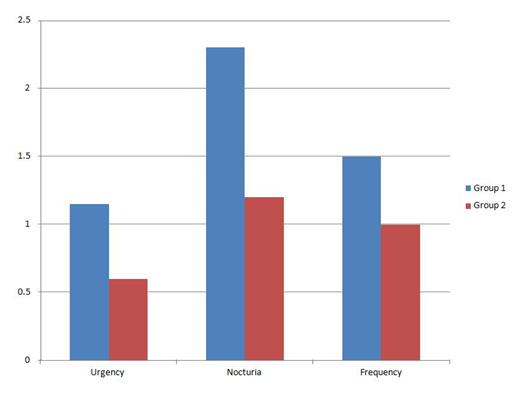
Frequency subscore was reduced significantly from baseline in both groups. Compared with group2 (mean of change -1±0.91), more reduction in IPSS frequency subscore was demonstrated in group1 (mean of change-1.5±1.27), but it was statistically not significant difference (P=0.180) as in Figure 4.
Side effects of Trospium chloride that observed were dry mouth in 10 patients (14.2%) and constipation in 2 patients (2.8%).
In urologic practice, storage (Irritative) symptoms are commonly seen; both in BPH and non-BPH patients.4 The first line treatment is usually one of the antimuscarinic agents.
In general, The treatment of BPH depends on α1 blocker agents & 5 reductase inhibitors, which are basically constructed to relieve obstruction, and there was a high precaution from using antimuscarinc drugs, but new studies reported an effective use of antimuscarinc agents for LUTS, without clinically significant effect on post voiding residual volume or increase risk of acute retention, especially when it is combined with an 1 blocker agent.10−12
Currently, many urologists worldwide are interested in using different antimuscarinc agents in combination with an blocker agent, seeking for optimal therapeutic effect.
This study was conducted to evaluate the use of the antimuscarinc agent (trospium chloride) for treatment of BPH symptoms.
We used Trospium chloride because it is a quaternary amine compound & Due to its low lipophilicity it had very limited passage to CNS so it has no negative effect on cognitive functions that is especially important in elderly patients ( like BPH patients ). Plasma half life is 20 hours & 60% excreted unchanged in urine, which may exert a local effect on bladder in addition to its systemic effect.10
It has a high and comparable binding affinity to M2 and M3 receptor subtype.
In addition, we were interested in evaluating Trospium chloride because no much studies available on its role in BPH/LUTS. And to be a controlled study, we divided our patients into two groups randomly & consequently; Group1 treated with tamsulosin & trospium chloride, and group 2 treated with tamsulosin alone.
We used both objective parameters (Qmax, PVR) & subjective parameters (IPSS/QoL) to evaluate the effectiveness & safety of the drug. Baseline parameters like age, prostate size and pre-treatment IPSS were comparable in the two groups, which exclude their effect on the results. There was a significant change in the IPSS, in both groups, in relation to baseline score, (Table 2) which reflects the effectiveness of both treatment arms, but there was no statistically significant difference in the mean of change between the 2 groups.
Parameter |
Group 1 |
Group 2 |
P.value |
||
|---|---|---|---|---|---|
Pre |
Post |
Pre |
Post |
||
IPSS |
17 |
8.82 |
18.4 |
10.2 |
0.909 |
QoL |
4.49 |
2.49 |
4.2 |
3 |
0.018 |
Obstructive score |
8.07 |
4.65 |
10.6 |
5.2 |
0.011 |
Q max |
12.8 |
15.6 |
15 |
17.28 |
0.81 |
Nocturia |
4.16 |
1.9 |
3.8 |
2.6 |
0.04 |
Urgency |
2.57 |
1.33 |
1.8 |
1.2 |
0.0941 |
Frequency |
2.24 |
0.74 |
2.2 |
1.2 |
0.18 |
PVR |
29.83 |
27 |
28.1 |
23.8 |
0.266 |
Table 2 The Mean value of different parameters, pre and post treatment in the two groups
The score of all the 3 irritative symptoms, dropped down in both groups, but the mean change was only significant for nocturia, and in favor of group 1(Table 1), while the difference in the mean change for frequency and urgency, though clearly present, but it was not significant between the 2 groups.
Such non significant changes between the 2 treatment groups, for frequency and urgency, were also found in the studies on Solifenacin, while studies on Tolterodine showed a significant difference in these parameters (Table 3).
Our study |
Lee KS et al.17 |
Kaplan SA et al.19 |
Kaplan SA et al.14 |
Chapple C et al.16 |
Yamaguchi O et al.15 |
|
Number |
71 |
228 |
664 |
398 |
62 |
638 |
Agent/dose agent |
TAM 0.4mg+TA M&TR 20mg |
Doxazosin ER(4mg)/propive rine (20 mg) + doxazosin ER (4 mg) |
Placebo/tolterodine ER(4mg)/tamsulosin(0.4mg)/both |
Solifenacin (mg) or placebo + tamsulosin (0.4 mg) |
Tolterodine ER (4 mg) or placebo+alpha-blocker |
Tamsulosin (0.2 mg) +solifenacin 2.5mw) &'placebo |
PVR (mL) |
+11.2vs7.6(P=0.266) |
+20.8 vs-4.7(P=0.002) |
-161vs ).27 vs 0.11vs 6.42; (NS) |
0.02 (0) vs- 13.5 (-8.0) |
116 vs 1.0 w: 0.0231) |
13.19vs 22.59 vs).92<0.001) |
Frequency |
-lvs-1.5 (P=0.180). |
-1.9 vs-0.9 (P=0.004) |
-1.4 vs-1.6 vs |
-1.05 vs -0.67 (P=0.135) |
-1.8vs- l.2 (P=0.0079) |
-1.27 vs-1.06 vs -0.22(P=<0.001) |
Urgency |
·0.6vs- 1.15) |
- |
-25 vs-2.7 vs -2.3vs-3.4 (P<0.0)) |
-2.18 vs- 1.10 (P<0.001) |
-2.9 vs-1.8 (P=0.0010) |
-2.18 vs-2.36vs -1.93 (NS) |
AUR |
None |
None |
3/220 vs V216vs 0/21) vs V22) |
7 (3%) vs 0 (0%) |
1.8%(6/329) vs 1.8%(6/323) |
1.9%(4/213) in solifenacin () mg) + |
Table 3 Comparisons between our studies and outcomes of five important randomized controlled trials
The significant difference in the mean change in obstructive symptoms collectively, was in favor of group 2 (Table 1), which may indicate some sort of less efficient voiding in the group treated with Trospium chloride, but this was only a subjective finding, while changes in the objective parameters of obstruction; PVR and Q max, were not significant between the two groups.
Changes in Q max were also not significant in many similar studies using different antimuscarincs like Tolterodine, Solifenacin and Propiverine, while changes in PVR, unlike ours, were significant in many studies using the above mentioned antimuscarinics (Table 2), which may indicate a more safer effect for TR over other antimuscarincs in this aspect.
Absence of acute retention of urine in our series is another proof for the safety of TR, and its relative superiority over other antimuscarincs that show an incidence of retention of urine up to 3% (Table 3).
The IPSS/QoL score was significantly decreased in group 1, in comparison with group 2 (Table 3), which mean a better QoL in the group treated with TR. This may be attributed to the significant decrease in nocturia in this group, which is one of the most bothersome symptoms of BPH.
This significant improvement in QoL score, was also mentioned in Kaplan et al study on Tolterodine , but it was not achieved in many studies using different antimuscarinics (Table 3).
During treatment course, most adverse events that possibly related to TR, were mild, and do not lead to withdrawal from the study. No patient suffered from AUR during treatment and no cognitive or visual disorder were reported in any patient , even dryness of the mouth related to Trospium was much less than in other antimuscarinics; which makes it more tolerable.
Trospium chloride, with its inhibitory effect on detrusor muscles was helpful in controlling the irritative symptoms especially nocturia, so that significantly improving QoL.
On the other hand, and for the same reason (inhibition of detrusor muscles), improvement in obstructive symptoms was lesser, but as there was no retention of urine reported, and no significant difference in the objective parameters of obstruction (PVR & Q max) in the 2 groups, we can consider it as a safe adjuvant treatment for BPH/LUTS.
Trospium chloride, when combined with the α blocker Tamsulosin, proved to be effective for patients with BPH/ storage LUTS which had a significant impact in improving their quality of life.
Trospium chloride also proved to be safe (no significant negative impact on voiding) and well tolerated by the elderly patients with BPH, as there was no adverse effect on cognitive or visual functions and low incidence of dryness of mouth.
None
Authors declare there is no conflict of interest.

©2018 Azzawi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.