eISSN: 2378-3176


Review Article Volume 7 Issue 3
Assistant Professor, Department of Medicine, Lady Hardinge Medical College and Associated Hospitals, India
Correspondence: Ramesh Aggarwal, Associate Professor, Department of Medicine, Lady, Hardinge Medical College and Associated Hospitals, New Delhi , Tel +919818626614
Received: April 24, 2019 | Published: May 24, 2019
Citation: Behera A, Aggarwal R. Renal manifestations in ?-Thalassemia patients. Urol Nephrol Open Access J. 2019;7(3):40-43. DOI: 10.15406/unoaj.2019.07.00241
Β-Thalassemia is among the common hemoglobinopathies which is frequently encountered in clinical practice. It affects not only the hematological system but affects other major important organ systems. Renal involvement in β-Thalassemia although not very uncommon but early symptoms like polyuria and mild proteinuria are missed in the questionnaire and clinical evaluation in follow up visits of the patients. The chronic anemia, iron overload and the chelating agents play the major factor for the renal impairment in these patients. Both tubular dysfunction and glomerular involvement is seen alongside the renal manifestation of the acquired infections during the course of illness. A high suspicion and clinical vigilance is essential for early diagnosis in these patients and a detailed investigation with necessary changes in management protocol may be required for avoiding advanced renal impairment in the patients of β-Thalassemia.
Keywords: β-Thalassemia, Thalassemia, Iron-chelation, proteinuria, tubular dysfunction, GFR, deferoxamine mesylate, deferipron, deferasirox, cooley’s anemia, anemia
β-Thalassemia is common among the hereditary hemoglobinopathies but with monogenetic inheritance and results from the mutation of the globin gene cluster situated on chromosome 11.1 Near about 300 mutations affects the synthesis of the β-globin chain. In β-Thalassemia major (Cooley’s anemia) there is absence of production of both the β-chains (β0/β0) and it manifest as severe microcytic and hyochromic anemia. The patient suffering from cooley’s anemia will require lifelong transfusion for survival. A asymptomatic variety may result which present with microcytosis and mild anemia (hemoglobin level, 70–90 g/L) and HbA2 level increases in which single β-globin chain production occurs (β0/β). Another entitity lies between the major and minor which presents with variable severity of anemia known as Thalassemia intermedia.2 Better health care facilities, judicious use of blood transfusion and availability of chelation therapies have made it possible for patients of β-Thalassemia to have prolonged life span and improved quality of life (QOL).3 As time passes ,the patient develops various morbidities, some related due to the disease and many related to the treatment the patient receives which are being more evident now-a-days due to improved life span. Various studies have proven the involvement of various organ systems but very little is available about the involvement of kidneys in the patients of β-Thalassemia. Chronic anemia, iron overload, infections (transfusion related and post splenectomy) and the drugs used for iron chelating have been postulated to be main factors for etio-pathogenesis of renal impairment.4
Pathophysiology
Urine formation involves the intricate balanced glomerular and tubular function. Every 24hrs, 180lts of water along with 24000mEq of Na, 700mEq of K, bicarbonate,20000mEq of chloride, proteins and monosaccharide’s undergo ultra filtration; but only 1 to 2litres 0f urine is produced with complete absorption of glucose and bicarbonate with secretion of selected molecules from the tubule. The selective tubular absorption and secretion is under the control of neurogenic mechanisms, hormones and enzymes.4
Tubular dysfunction (Figure 1)
Although it has been ascertained that the chronic anemia in β-Thalassemia and the chelators of iron used for iron overload following repeated blood transfusion are the major cause for the renal impairment but the independent contribution of each factor needs to be investigated in details. Manifestation of epithelial tubular cell damage largely depends on the site of the injury of the tubule and the severity of the cellular damage, manifesting only as increased loss of tubular proteins and changes in change in electrochemical gradient across the cells to severe ATN, non anionic gap acidosis and fanconi syndrome. In almost all patients of β-Thalassemia low molecular weight proteinuria is present. Moreover, several studies report in a majority of patients with β-Thalassemia there is increased loss of biomarkers in urine suggesting proximal tubular damage. The various biomarkers include N-acetyl-β-D-glucosaminidase and β2-microglobulin (may be up to 60%). The other molecules which points towards the tubular damage include calcium (approx 13%), phosphate and magnesium (9%), uric acid (30-40%), malondialdehyde (from peroxidation of membrane lipids) and other amino acids (approx 30%).4 The β-Thalassemia major patients who have undergone stem-cell transplantation as curative treatment have significant reduction of secretion of markers in comparison to patients who have not undergone transplantation in age matched population.5 The mechanism of injury in proximal tubular cells is mediated by mitochondrial stress. The mitochondrial injury is quite evident by the increased efflux of cytochrome C, lactate dehydrogenase and fall in ATP (Adenosine triphosphate). Autopsy findings have shown an increased deposition of iron in the renal tubular cells (distal as well as proximal) and the acidic environment of specifically the proximal tubular cellar mileau helps in dissociation of iron from transferring and increased free radical production resulting in injury to the brush border epithelium.6–11 Clinical studies have shown a close correlation between the markers of tubular toxicity levels and the serum ferritin levels and further, the response of chelation therapy on reversal of tubular defects.12 A good correlation has been proven between the chronic anemia with that of the markers of oxidative injury, lipid peroxidation of the cellular membrane and functional impairments of the tubular cells.13
Glomerular filtration rate abnormalities (GFR) (Figure 1)
In β-Thalassemia patients, GFR abnormalities are also quite evident manifesting more profoundly in patients of β-thalassemia intermedia who do not require regular blood transfusions and they manifest as increased glomerular filtration rate and creatinine clearance more than the general population.14 The increase in GFR can be explained by the underlying anemia. The glomerular hyper perfusion and the hyper filtration leads to glomerular capillary wall stretching, resulting in endothelial and epithelial dysfunction and injury resulting in transudation of the macromolecules into the mesangium which leads to Mesangial dysfunction and decline in GFR in the long run.15 Hypoxia(due to anemia and increased metabolic need) leads to tubular cell loss by apoptosis or epithelial- mesenchymal transdifferentiation which leads to tubulo-interstitial injury and sclerosis of glomerulo.16 Excess iron causes peroxidation of the tubular cellular membrane causing serious damage of the tubular cells and in response to it the cytokines and growth factors are released that can cause glomerular sclerosis and tubule-interstitial scarring with subsequent fall in GFR.17 The other factors that lead to decline in GFR are the infections, whether related to blood transfusions like Hepatitis C virus, hepatitis B virus, and Human immunodeficiency virus (HIV) infections or the higher propensity for other infections due to chronic anemia, decreased immune response and iron overload which leads to various types of glomerulonephritis.18 The glomerular dysfunction also occurs due to intake of NSAID and use of angiotensin-converting enzyme blockers and angiotensin receptor blockers for associated proteinuria or hypertension present. Similarly, liver failure and cardiac failure, due to multiple transfusions resulting in iron overload in Thalassemia major, is one of the important cause for systemic as well as renal abnormal hemodynamic changes that may eventually lead to renal hyproper fusion and fall in GFR.19
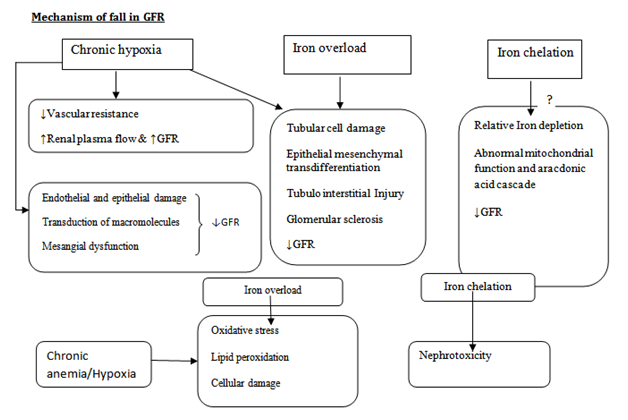
Figure 1 Mechanisms of renal disease in patients with β-thalassemia.21
Iron chelators and nephrotoxicity
For the management of Iron overload following repeated blood transfusions, three iron chelators are used commonly:
Acute kidney injury due to the drugs is although rare but has been reported most commonly due to deferoxamine overdose due to instrumental failure during infusion or inadequate monitoring during infusion which resulted in acute renal failure sometimes requiring dialysis. Few cases of acute renal failure have been reported with Deferasirox which is oral iron chelators in its post-marketing surveillance.4 The increase in serum creatinine with Deferasirox was usually transient and never exceeded twice the normal range and in a dose-dependent manner. The increased acute kidney injury risk with high-dose Deferasirox and low serum ferritin concentration points towards over chelation as a causative factor for the AKI.20 With the use of iron chelators three basic questions needs to be primarily assessed for renal dysfunction as to ascertain the true reason for renal dysfunction which are as follows4
A simplified recommendation has been put forth with regards to use of Deferasirox therapy with changes in renal impairment4 (Table 1).
|
Monitoring(GFR) |
· Before initiation and monthly thereafter. |
|
· Elderly/Using other medicinal products-Weekly in |
|
|
1st month then monthly |
|
|
>33% above pretreatment values at two consecutive visits (not attributed to other causes) |
Deferasirox dose should be reduced by 10mg/kg |
|
Progressive increases beyond the upper limit of normal. |
Deferasirox should be interrupted, then reinitiated at a lower dose followed by gradual dose escalation if the clinical benefit outweighs the potential risks |
|
In pediatrics population, >33% above pretreatment values and above the age-appropriate upper limit of normal. at two consecutive visits |
Deferasirox dose should be reduced by 10mg/kg |
Table 1 Monitoring and dose modification of iron chelators according to GFR
Nephrolithiasis
The various factors that increase the incidence of renal stones include non judicious use of vitamin D, calcium supplements and Deferasirox, Tubular dysfunction increases the risk of calcium and cysteine stones. Splenectomy which increases the cell turover increasing the risk of uric acid stones.
Investigations
Apart from the urine microscopy for proteinuria, casts and evidence of active sediments for acute injury to the glomerular and tubular components of the kidney newer biomarkers have been used to diagnose early renal injury without an invasive approach like renal biopsy. The various biomarkers include serum β2-microglobulin, N-acetyl-beta-D-glucosaminidase, neutrophils gelatinase-associated lipocalcin are showing promising result for early diagnosis in diagnosing of renal impairment in β-Thalassemia.21,22
Dysfunction of tubules and mild glomerular abnormalities reflecting as GFR abnormalities are not uncommon in patients with Thalassemia. Chronic anemia, iron overload following repeated blood transfusion due to repeated blood transfusions and over chelation are the leading factors for the pathogenesis of renal impairment. Patients are unable to maximally concentrate the urine due to renal tubular dysfunction and present with polyuria in early stages. The use of iron chelators may sometimes manifest with elevated creatinine levels which are commonly no progressive and resolve without any interventions or modifying the chelating dose. Over-chelation and over-zealous sudden fall in iron levels are also to be avoided. A strong suspicion and clinical vigilance is the key for early recognition and evaluation for the cause of renal impairment in the patients of β-Thalassemia.
None
The authors declared there is no conflict of interest.

©2019 Behera, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.