eISSN: 2378-3176


Case Report Volume 2 Issue 5
University Medical Unit, National Hospital of Sri Lanka, Sri Lanka
Correspondence: Weerakkody RM, University Medical Unit, National Hospital of Sri Lanka, Sri Lanka, Tel 447424980393
Received: August 03, 2015 | Published: December 30, 2015
Citation: Weerakkody RM, Sheriff MHR. Primary hyperoxaluria: a Sri Lankan case series. Urol Nephrol Open Access J. 2015;2(5):175-178. DOI: 10.15406/unoaj.2015.02.00059
Introduction: Primary hyperoxaluria is an extremely rare metabolic disorder with autosomal recessive inheritance. We report the first case series in Sri Lanka which comprises of five patients, referred for the management of nephrocalcinosis or end stage renal failure.
Case presentation: Cohort consisted of five patients of Asian origin, three males, with a mean age of 26 (range 15-42). They had mean serum creatinine level of 473µmol/l (range 78-1435). Mean age of first symptom was 15.4years (range 8-26). Primary hyperoxaluria type 1 was diagnosed, and confirmed genetically in all of them. One subject, who had a rare mutation, had normal urinary excretion of oxalate, and was a product of a consanguineous marriage, oxalosis involving heart, bone, vessels and joints were observed in three subjects. One developed complete heart block, a novel finding, as a result of severe cardiac oxalosis. Primary hyperoxaluria type 1 is commoner than types 2 and 3, and is due to defective enzyme activity of the liver, resulting in overproduction of oxalic acid. Diagnosis is by estimation of urinary oxalate excretion, but false negatives are common in end stage renal disease. The cohort of patients developed end stage renal failure in early 20s, similar to previous series. Maintaining high urine output, low oxalate diet, oral citrate and high dose pyridoxine are mainstay of conservative treatment. Definitive treatment is liver transplantation, and liver-kidney in the case of end stage renal failure.
Conclusion: Primary hyperoxaluria is a notoriously difficult disease to diagnose and manage. Even after successful renal transplant, oxalate deposition can damage the allograft.
Keywords: nephrocalcinosis, hyperoxaluria, cardiac oxalosis, complete heart block, liver-kidney transplantation
Primary hyperoxaluria is a rare metabolic disorder with autosomal recessive inheritance. Three mutations have been described leading to primary hyperoxaluria types I, II and III. The metabolic defect in primary hyperoxaluria Type I, described as mutation of alanine-glyoxylate aminotransferase, which is found only in the hepatic peroxisomes. Pyridoxine (vitamin B-6) is a cofactor in this chemical pathway. Primary hyperoxaluria Type II, the defect is in D-glyceric dehydrogenase. Oxalate is excreted in the urine, which leads to nephrocalcinosis and the eventual development of end-stage renal failure.1 Deficiency of 4-hydroxy-2-oxoglutarate aldolase cause Primary hyperoxaluria Type III.2 We report the first case series in Sri Lanka which comprises of five patients, presented to the University Medical Unit of National Hospital.
Table 1 summarized the basic demographic and biochemical information about the five cases. All were of Asian origin and specific differences of each case are described below.
|
RR |
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
Means |
Age (y) |
|
21 |
35 |
42 |
15 |
17 |
26 |
Gender |
|
Female |
Male |
Male |
Male |
Female |
|
Age of First Symptom |
|
16 |
18 |
26 |
9 |
8 |
15.4 |
Serum Creatinine (µmol/l) |
< 150 |
510 |
1435 |
201 |
140 |
78 |
473 |
Serum Ionized Ca2+ (mmol/l) |
1.1 – 1.35 |
1.12 |
1.04 |
1.20 |
1.17 |
1.21 |
1.15 |
Serum PO43- (mmol/l) |
0.74-1.52 |
1.6 |
1.16 |
0.81 |
1.4 |
1.05 |
1.20 |
Urinary Ca2+ Excretion (mmol/d) |
2.5-7.5 |
|
0.45 |
0.28 |
1.7 |
1.6 |
1.01 |
Urinary PO43- Excretion (mmol/d) |
16-48 |
|
22 |
16 |
30 |
34 |
25.5 |
Urinary Oxalate Excretion (mmol/d) |
0.23-0.68 |
0.44 |
23 |
11 |
11 |
11.7 |
11.4 |
Table 1 Basic demographic and biochemical information of the cohort for comparison and contrast. Note the wide range of 24h urinary oxalate levels observed
Case 1
A 21year old female, presented with nephrocalcinosis and in end stage renal failure. After assessment for causes for nephrolithiasis, and with a high normal 24hour oxalate excretion she underwent live related kidney transplant. After 4months of the transplant her creatinine levels started rising. Renal biopsy demonstrated crystal induced tubulitis. A genetic analysis confirmed primary hyperoxaluria type I, and showed an 8 base pair deletion (c.447_454del-GCTGCTGT) in AGXT gene. Her graft function continued to deteriorate and after 19months of post transplant, she developed end stage renal failure, and commenced on hemodialysis. Her condition was complicated with features of systemic oxalosis; recurrent fistula thrombosis; cardiac oxalosis, presenting as diastolic dysfunction; oxalate arthropathy; and severe osteopenia. She underwent liver transplantation, but sadly passed away due to complications.
Cases 2 & 3
A 35year old male with nephrocalcinosis was referred to us in end stage renal disease. He had elevated 24 hour urine oxalate levels. He was started on hemodialysis, but the requirement of 6 hour daily dialysis could not be met for effective oxalate clearance. Instead he was on twice weekly dialysis, with Kt/V > 1.3. He developed near complete heart block during inter-dialysis period, which needed temporary pacing. The electrolyte profile was normal (K+-5.2, Na+-142, ionized Ca2+-2.3, Mg2+-1.3, all in mmol/l) during the episode. Echocardiography showed increased echogenicity of myocardium suggestive of oxalate cardiomyopathy. The coronary angiography was normal. Unfortunately the patient refused an endomyocardial biopsy to confirm the diagnosis of cardiac oxalosis. With intensified dialysis (four times weekly), he improved his symptoms. Later he developed oxalate arthropathy which affected wrist and small joints of the hand and was managed symptomatically.
The above subject’s brother (42) was diagnosed during family screening; had a serum creatinine of 150µmol/l; diagnosed of nephrocalcinosis; and had elevated 24 hour oxalate excretion. He was suffering from calculus disease since 15years of age, with many episodes of renal colic. At the age of 31 he developed obstructive calculus of the left kidney which led to hydronephrosis. He underwent emergency decompression with percutaneous nephrostomy, followed by anterograde double J stenting. Three months later he underwent extra corporeal shockwave lithotripsy which cleared the obstruction and resolved hydronephrosis. Both of them had their renal stones analyzed. The 35 year old patient had a 497mg-calculi, which consisted of 313mg of oxalate and 88mg of calcium. His brother had a 1582 mg-calculus, which contained 867 and 231mg of oxalate and calcium respectively. Both have undergone genetic analysis, which confirms primary hyperoxaluria type I, with a point mutation NM30000.2 (AGXT): c1095 G>A; p.G365D.
Case 4
A 15 year old male was referred from pediatrics unit for further management of nephrocalcinosis. He had a serum creatinine level of 140umol/l with elevated 24 hour urine oxalate excretion. He also has had many episodes of renal colic, but never had features of hydronephrosis or obstructive uropathy. He currently has no features suggestive of systemic oxalosis, with good cardiac function and absence of joint symptoms.
Case 5
A 17 year old female was referred from Pediatrics unit for further management of recurrent nephrolithiasis and nephrocalcinosis. She is active and other than for few episodes of renal colic, is asymptomatic, and has a creatinine value of 78umol/l. There are no features of oxalosis clinically or radiologically. Cases 2-5 were tested positive for the same mutation. All of them were products of non-consanguineous marriages, and had no family history of renal disease. Although the mutations are identical, they are likely to be de novo mutations.
Primary hyperoxaluria is due to a genetic defect that causes a loss of specific enzymatic activity, resulting increased oxalate production through alternate pathway. There are three types described, transmitted with autosomal recessive inheritance. Primary hyperoxaluria type 1 is the commonest, with an incidence of 1 per 120,000 live births. Hyperoxaluria type 1 is due to defect of alanine-glyoxylate which detoxifies glyoxylate. Pyridoxine is a cofactor in this chemical pathway, and forms the basis of treatment of primary hyperoxaluria type 1. Hyperoxaluria leads to nephrocalcinosis and the eventual development of end-stage renal failure, usually in childhood. In a retrospective cohort study of 155 patients with primary hyperoxaluria type 1, pGly170Arg AGXT mutation was the most common and was associated with a better prognosis. The median age at end-stage renal disease was 47years in pGly170Arg homozygote and 35years in heterozygote. Among patients with other mutations, the median age at end-stage renal disease was 21years.3 Primary hyperoxaluria type 2 less common than type 1, and the defective enzyme is D-glyceric dehydrogenase. Pyridoxine is not effective. Hyperoxaluria type 3 is due to deficient of 4-hydroxy-2-oxoglutarate aldolase.2
The median age at initial symptoms of type 1 is 4 to 7years in Europe and 13years in Japan, ranging from birth to the sixth decade. In this series it was 15.4years of age. Primary hyperoxaluria type 1 has variable presentation; an infantile form; recurrent urolithiasis and progressive renal failure in childhood or adolescence; and late-onset form with occasional stone passage, and end stage renal disease.3 Symptoms of primary hyperoxaluria type 2 occurs late in childhood, form less calculi, have a less pronounced nephrocalcinosis, and a lower incidence of end stage renal disease over time, compared to primary hyperoxaluria type 1.3 Interestingly, in our case series patients have developed end stage only in their early third decade. Mutation carried by Case 1 is rare among Caucasians, but commoner in Asians. The onset of end stage renal disease was delayed - or yet to happen as in subjects 4 and 5 - although they carried a mutation commonly found in Caucasians.4
Systemic deposition of oxalate, namely, oxalosis, can then occur in many organs such as bone, retina, skin, soft tissues, heart, vessels, and central nervous system. Systemic oxalosis is thought to take place once the GFR falls below 30-50ml/min.3 First patient showed extensive bone involvement due to oxalosis (Figure 1 & 2), which resulted in severe bone pain and radiological changes. Additionally she had fistula issues with recurrent thrombosis and hemorrhage from puncture sites, which is a known vascular complication.5 She was unfortunate to miss the diagnosis presenting with high normal 24hour oxalate excretion-at her presentation and then to receive a renal transplant, only to see it failing rapidly. It is a well recognized diagnostic and management issue when patient first presents in end stage renal failure.6,7 The diagnosis was only apparent after the genetic testing, which is not affordable for many of the sufferers of disease.
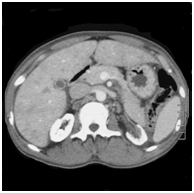
Figure 1 Abdominal X-ray of subject 2, showing a left sided upper ureteric calculus with the stent in situ. Calcified left kidney outline is visible, while the right sided kidney appears radio-opaque due to heavy deposition of calcium oxalate. Note the high density of the bones due to oxalate deposition (bone oxalosis).
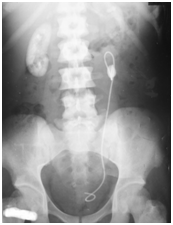
Figure 2 Non contrast enhanced computed tomography of the abdomen of subject 2. Note the bone-identical density of the renal parenchyma due to oxalate deposition.
The second patient had cardiac oxalosis, presenting as diastolic heart failure and rhythm disturbances. Although cardiac oxalosis and rhythm disturbances have been described in literature,8 complete heart block has not been previously described in patients with suspected cardiac oxalosis (Figure 3). The cases 4 and 5 are at relatively early stages of the disease. They have been provisionally diagnosed with the condition following 24hour oxalate excretion and the appearance of their kidneys on imaging. The genetic testing confirmed the diagnosis. Conservative management consists of Pyridoxine, Citrate, high fluid intake and normal calcium diet.3 The curative treatment is combined liver-kidney transplantation. The outcomes are ever improving, and graft loss due to oxalosis has been insignificant.9
Primary hyperoxaluria is a rare inborn metabolic error, which is notoriously difficult to diagnose in resource poor environment. It needs early detection, since late stages can only be managed with liver-kidney transplants and in established oxalosis, the recipient may not even be fit for a transplant. Sri Lankan patients develop end stage renal disease relatively late.
None.
Author declares that there is no conflict of interest.

©2015 Weerakkody, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.