eISSN: 2378-3176


Research Article Volume 7 Issue 6
1Locum Consultant Urologist in NHS – UK
2Physical activity and health promotion, Department of Biomedicine and prevention, Faculty of Medicine and Surgery, University of Rome Tor vergata, Italy
3Urology Fellow, Western General Hospital, UK
4Consultant Urologist; National Institute of Urology and Nephrology, Egypt
Correspondence: Abdalla Ali Deb, Locum Consultant Urologist in NHS – UK
Received: November 29, 2019 | Published: December 9, 2019
Citation: Deb AA, Okechukwu CE, Emara S, et al. Occupational exposure as risk factor for kidney and bladder cancer: a systematic review and meta-analysis. Urol Nephrol Open Access J. 2019;7(6):143-151. DOI: 10.15406/unoaj.2019.07.00261
Background: Occupational exposure plays a huge role in the epidemiology, pathogenesis and prevalence of kidney cancer (KCa) and bladder cancer (BCa) worldwide.
Objective: The aim of this study was to analyze qualitatively and quantitatively the association between occupational exposure and risk of KCa and BCa.
Method: We identified peer-reviewed articles published in English by searching PubMed, Embase, Surveillance, Epidemiology, and End Results (SEER) and Cochrane databases. We selected articles published between January 2018 to May 2019. We summed up all relative risk estimates to ensure accuracy, comprehensiveness and maximize statistical power given the low absolute occupational risk of KCa, occupational risk of BCa and standardized incidence ratios (SIRs). We reported this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) checklist. Quantitative analyzes was performed using Comprehensive Meta-Analysis version 3 (Biostat, Inc, Englewood, New Jersey, USA).
Results: We found no significant association between occupational exposure and KCa (OR 1.04, 95% CI: 0.94-1.34), but there was a significant association between occupational exposure and BCa (OR 1.54, 95% CI: 1.44-1.75).
Conclusion: From the result of our qualitative and quantitative analysis there was no significant association between occupational exposure and KCa whereas there was a significant association between occupational exposure and BCa.
Keywords: carcinoma, renal cell, kidney neoplasms, urinary bladder neoplasms, occupational exposure, neoplasms
KCa, kidney cancer; BCa, bladder cancer; SEER, surveillance, epidemiology, and end results; SIRs, standardized incidence ratios; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCC, renal cell carcinoma; CI, confidence intervals; SIRs, standardized incidence ratios; OR, odd's ratio
Occupational exposure continues to play a huge role in the epidemiology and pathogenesis of cancer worldwide, the prevalence of kidney cancer (KCa) and bladder cancer (BCa) in France is heavily attributed to occupational hazards and exposure to industrial chemicals.1 Exposure to industrial chemicals like amines, chromates, dinitrotoluene, arsenic, beryllium, cadmium, nickel, wood dust, crystalline silica, brown coal phosphors, furnace emissions, smoke from diesel engine, ionizing radiation and non-ionizing radiation, thermal shock Asbestos and aniline are major occupational risk factors of KCa and BCa, also regular shift work, stress and sedentary job contributes to the development of KCa and BCa.2 Exposure to perchloroethylene mainly used for dry cleaning of fabrics by dry cleaners was associated with KCa.3 The risk of renal cell carcinoma (RCC) was significant in aircraft mechanics, shipbuilders and painters, who were exposed to industrial chemicals.4 Exposure to pesticide among farmers may have significant association with kidney cancer.5 Traces of heavy metals such as Nickel (Ni) , Chromium (Cr) ,Iron (Fe), tungsten (W), titanium (Ti), Cadmium (Cd), Copper (Cu), Manganese (Mn), Zinc (Zn) particles were found in the analysis of tumor tissue samples using spectrum of X-ray microanalysis.6 This shows that heavy metals plays a vital role in the pathogenesis of cancer, Welders are frequently exposed to these heavy metals, and this suggests that they are highly predisposed to KCa and BCa.6
KCa was ranked the ninth and fourteenth common cancer cases in men and women, respectively.7 Apart from occupational exposures, environmental exposures and behavioral factors are associated with the development of KCa.8 Highly skilled workers in some occupation that involves little or no exposures to stress and chemicals often have less chances of developing RCC.9 Consumption of fruit, vegetables, and alcohol was linked with a decreased risk of RCC despite the presence or exposure to the risk factors.10 High levels of arsenic in the urine was associated with the pathogenesis KCa.11 Exhaust from diesel engine is associated with the pathogenesis of RCC.12 KCa is prevalent in the United States due to occupational exposures.13 BCa is prevalent worldwide, Exposure to heavy industrial chemicals like trichloroethylene, perchloroethylene, aromatic hydrocarbon solvents, benzene and toluene in the working environment and cigarette smoking are the main risk factors of BCa.14 Kishor et al.8,15 observed statistically significant increased risks of BCa among tobacco workers, plumbers, chimney sweeps, waiters, hairdressers, seamen, printers and they observed Significant decrease in risks among farmers and forestry workers. There is need to critically analyze and review the occupational risk factors associated with KCa and BCa for the purpose of primary prevention and maintaining occupational safety and health. The aim of this study was to analyze systematically and quantitatively the association between various occupational exposures and risk of KCa and BCa.
We performed and reported this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) checklist.16
Search strategy
We identified peer-reviewed articles published in English by searching PubMed, Embase, Surveillance, Epidemiology, and End Results (SEER) and Cochrane databases. We selected articles published between January 2018 to May 2019. The following search terms was generally used; KCa, renal cancer and occupational exposure, BCa and occupational exposure, neoplasms, and tumor. First, we screened all titles and abstracts we read the full text of relevant studies. We selected all available studies related to occupational exposure in relation to KCa and BCa. With regards to MeSH terms and relevant keywords (Carcinoma, Renal Cell, Kidney Neoplasms, Urinary Bladder Neoplasms, Occupational Exposure, Neoplasms), we used the Cochrane Highly Sensitive Search Strategy for identifying reports of articles in PubMed. We restricted the search to articles published in English.
Study eligibility
Studies were included if: 1. they evaluate occupation exposures as risk factors for KCa and BCa 2. They were designed as cohort, case-control, cohort-case control, cross-sectional, or ecological studies 3. They provided data on KCa and BCa risks associated with occupation exposures. Studies were excluded if: 1. they were not conducted on humans 2. They were designed as individual case reports 3. They did not contain original data and instead provided reviews, reanalysis, or commentaries and 4. They did not analyze the role of occupational exposures in the pathogenesis of KCa and BCa.
Data extraction
We extracted the following data of each eligible study onto a spreadsheet: first author, publication year, location, study type, follow-up period, study population, sample size, type of occupational exposure, numbers of KCa cases reported, number of BCa cases reported, association measures and their 95% confidence intervals (CI), and controlled confounding factors.
Statistical analysis
We summed up all relative risk estimates to ensure accuracy, comprehensiveness and maximize statistical power given the low absolute occupational risk of KCa; occupational risk of BCa, standardized incidence ratios (SIRs).We applied Q test and I2 index to evaluate heterogeneity across studies. Q test P values of less than 0.1 indicate significant heterogeneity, and I2 values of near or less than 25%, near 50%, and near or higher than 75% represent low, moderate, and high heterogeneity, respectively. We performed analyzes based on study designs and populations to investigate possible sources of heterogeneity. We made use of the following tools: 1. Pooled OR: for analysis of multiple risks and Odd's ratio (OR) of multiple studies, and found adjusted accumulative OR for all studies 2. Fisher’s method: Combining p values from independent tests bearing upon the same overall hypotheses 3. Z score method: to test difference in mean 4. Test for heterogeneity: Cochran’s Q test and I2: Under null, it is approximately, distributed as a chi-square with k-1 degrees of freedom for test heterogeneity and homogeneity of studies results and finding. All analyzes were performed using Comprehensive Meta-Analysis version 3.3.070 (Biostat, Inc, Englewood, New Jersey, USA).
Study selection
The search yielded 15,110 articles in total. After removing duplicates, 7,569 articles remained. We excluded 2,340 articles because their abstracts were irrelevant with regards to the topic of our study. The references of the 5229 remaining articles were searched for full-text reviews. We excluded 1254 studies with unclear results, 1986 reanalysis studies, 1392 studies that did not report the outcome of occupational exposure as risk factors for and BCa, 461 studies with inadequate evidence and 120 study with unavailable full text. The remaining 19 studies were systematically reviewed, while 16 studies were included for meta-analysis (Figure 1).
Study characteristics
As shown in Table 1 and Table 2, the studies that was selected for the systematic review and meta-analysis were published between January 2018 and May 2019. We qualitatively analyzed these articles in order to ascertain the association between occupational exposure and risk of KCa and BCa.
Study |
Country |
Study type |
Follow-up |
Study population |
Sample size |
Case |
Increased risks (95% CI) |
Outcome |
Michelek et al.17 |
Finland, Iceland, and Sweden |
Case-control study |
1960–1990 |
population censuses |
59,778 KCa cases, and 298,890 controls |
59,778 |
Individuals with high exposure to asbestos (OR 1.19, 95%CI 1.08–1.31). |
High risk of KCa was observed among individuals exposed to welding fumes. |
Michelek et al.18 |
Nordic population |
Case-cohort study |
1960-1990 |
population censuses |
14.9 million |
59,778 |
SIRs: welders [1.24, (95% CI) 1.14 to 1.35], public safety workers (1.16, 95% CI 1.08 to 1.25). Seamen (1.16, 95% CI 1.07 to 1.26). |
Variation in the incidence of KCa was observed. |
Zaitsuet al.19 |
Japan |
Case-cohort study |
1984 to 2016 |
Nationwide inpatient records |
3316 cases of renal cell cancer (excluding upper tract urothelial cancer) and 168 418 controls. |
3316 |
(OR, 1.61; 95% CI, 1.34‐1.93). |
Occupational class is associated with the risk of KCa in men. |
Peters et al.20 |
Canada |
Case-control study |
1994 and 1997 |
National Enhanced Cancer Surveillance System |
712 cases and 2454 controls |
712 |
(OR 1.2, 95% CI, 1.0–1.4). |
High level of exposure to asbestos is associated with KCa risk |
Peters et al.21 |
Canada |
Case–control study |
1994–1997 |
National Enhanced Cancer Surveillance System (NECSS) |
712 cases and 2457 cases |
712 |
(OR diesel=1.23, 95% CI=0.99–1.53; OR gasoline=1.51, 95% CI=1.23–1.86). |
Occupational gasoline and diesel exhaust exposure may increase the risk of KCa. |
Saint-Jacques et al.22 |
Canada |
Ecological study |
1998-2010 |
inpatient records- Nova Scotia, Canada |
864 bladder and 525 kidney cases |
864 |
- |
This study suggests an increased KCa associated risk from drinking water with increased arsenic-levels |
Michalek et al.23 |
Denmark Iceland |
Cohort study |
- |
National censuses |
14.9 million |
59,778 |
The highest SIRs were found in seamen (1.51, 95% confidence interval [CI] 1.23–1.82), printers (1.39, 95% CI 1.11–1.71), welders (1.37, 95% CI 1.03–1.78), and public safety workers (1.35, 95% CI 1.12–1.62). |
There was an association between profession and risk of malignancy of the renal pelvis. |
Callahan et al.24 |
USA |
Case-control study |
2002 and 2007 |
Lead industry workers |
1217 cases and 1235 controls cases and 1235 controls |
1217 |
(OR 0.9, 95% CI 0.7 to 1.3 for highest quartile vs unexposed; ptrend=0.80). |
No association was found between occupational lead exposure and KCa |
Table 1 Occupational exposure and risk of kidney cancer
Cis, confidence intervals ORs, odds ratios SIRs, standardized incidence ratios
Study |
Country |
Study type |
Follow-up |
Study population |
Sample size |
Case |
Increased risks (95% CI) |
Outcome |
Carey et al.25 |
Denmark Finland Iceland Norway Sweden |
Cohort study |
1961–2005 |
Nordic Occupational Cancer study (NOCCA) |
111,458 |
111,458 |
(SIR 1.29, 95% CI 1.05–1.56), waiters (1.22, 1.07–1.38) hairdressers (1.14, 1.02–1.26), cooks and stewards (1.12, 1.01–1.25), printers (1.11, 1.04–1.18) and seamen (1.09, 1.03–1.14). |
Smoking is a strong risk factor for BCa. |
Turner et al.26 |
Spain |
Case-control study |
1998–2001 |
Large‐scale Spanish BCa Study. |
938 cases and 973 controls |
938 |
(OR per 5.9 μg/m3=1.06, 95% CI 0.71–1.60) or NO2 (OR per 14.2 μg/m3=0.97, 95% CI 0.84–1.13) |
There was no clear evidence for associations of ambient particulate matter (PM2.5) and nitrogen dioxide (NO2) concentrations and incident BCa risk. |
Makiko et al.27 |
Japan |
cross-sectional study |
2017 |
76 ortho-toluidine (OT) and/or aromatic amine-exposed workers |
76 |
10 |
- |
OT-exposed workers had histories of gross hematuria and cystitis. |
Marant et al.28 |
France |
Cohort study |
2015 |
Surveys among employees, the national labor force data, a cohort of agricultural workers, national monitoring of workers |
7905 |
7336 among men and 569 among women |
- |
BCa was ranked as the 3rd occupation associated cancer in men. |
Carta et al.29 |
Italy |
cross-sectional study |
- |
Non-Muscle-Invasive Bladder Cancer (NMIBC) patients |
160 |
160 |
(HR=0.195; 95% CI=0.060 to 0.623; p=0.006). |
A mechanism exist between cause (ValVal genotype of both MnSOD and COMT) and effect (decreased progression of tumor in NMIBC patients). |
Régis et al.30 |
France |
A nested case–control study |
2006 to 2012 |
Cohort of workers from six French steel-producing factories |
Cases (n=84) and controls (n=251) |
84 |
(OR=1.13 (1.02–1.25) |
There is an increased risk of BCa observed among workers exposed to straight metalworking fluids (MWFs) |
Sciannameo et al.31 |
Italy |
case–control study |
1992-2012 |
chemical engineering technicians postmen lathe operators |
893 cases and 978 control. |
893 |
- |
A significantly increased BCa risk was found for chemical engineering technicians, postmen, and lathe operators. |
Jung et al.32 |
Canada |
societal perspective |
2011 |
Diagnosed cases of BCa in Canada that is associated with occupational exposure |
199 |
199 |
- |
The economic burden of BCa due to occupational exposures is very enormous |
Noon et al.33 |
Finland |
Cohort study |
- |
National data set of workers |
1.7 million Finnish men, 1.7 million women |
13 717 with BCa for men, 4282 with BCa for women |
Male chemical process workers (SIRloc/SIRadv: 5.19; 95% CI, 1.73–25.7), male military personnel (SIRloc/SIRadv: 6.4; 95% CI, 1.09–259.0), male public safety workers (SIRloc/SIRadv: 1.77; 95% CI, 1.04–3.23), Miscellaneous construction workers (male SIRloc/SIRadv: 0.67; 95% CI, 0.53–0.86; female SIRloc/SIRadv: 0.12; 95% CI, 0.09–0.54) |
Occupations may differ in their risks for localized and advanced BCa. |
Golka et al.34 |
Germany |
case–control study |
July 2009 to July 2013 |
Former hard coal miners |
400 bladder cancer cases and 442 controls |
400 |
(OR 0.96, 95% CI: 0.73 to 1.26; smokers: OR 0.93, 95% CI: 0.66 to 1.30; non-smokers: OR 1.02, 95% CI: 0.58 to 1.80) OR 3.22, 95% CI: 1.39 to 7.49); hospital B: 32 cases (16%) and 20 controls (10%) (OR 1.72, 95% CI: 0.95 to 3.12) |
There was an elevated BCa risk in former hard coal miners |
Hameed et al.35 |
Egypt |
case–control study |
- |
Patients from different upper Egypt governorates |
100 cases, 200 controls |
100 |
- |
The results revealed that the level of P53 was significantly high in comparison with the control group (p < 0.001). |
Table 2 Occupational exposures and risk of bladder cancer
Cis, confidence intervals ORs, odds ratios SIRs, standardized incidence ratios
Quality assessment of studies
For the studies that investigated the association of occupational exposure and risk of KCa; there were 4 case control studies,17,20,21,24 3 cohort studies18,19,23 and 1 ecological study22 they were all population based studies. For the studies that investigated the association of occupational exposures and risk of bladder cancer; there were 5 case control studies,26,30,31,34,35 3 cohort studies,25,28,33 2 cross sectional studies,27,29 and 1 social perspective.32 All studies that reported on occupational exposure as risk factors for KCa and BCa Between January 2018 to May 2019 was initially selected for the study. To assess the quality of the studies, STROBE36 was used as the standard checklist. This checklist contains 22 items that cover different parts of a report (sampling, measurement of variables, study objectives, and statistical analysis). We gave one point to each item, and some other items that were more important to us had more points. The STROBE checklist contains 22 sections that cover different parts of a report, and the maximum score of a report equals 44, so that a score of 1–15 indicates poor quality, 16–30 shows average quality, and 31–44 is considered to be excellent. We excluded 3 articles with an overall score less than 16 for the meta-analysis.
Risk of bias within studies
Association and risk of occupational exposures for kidney cancer distribution
To assess publication bias a funnel plot was derived, and heterogeneity among studies results was checked. Confounding, interaction, and bias account for differences in results among studies, which are not due to chance, after quantifying, we discovered that it contributed to heterogeneity among these studies. We found significant heterogeneity and disagreement between studies (Table 3). Heterogeneity and asymmetry was illustrated in funnel plot (Figure 2).
Test for heterogeneity |
|
Cochran Q |
40.32 |
P |
0.00** |
I2 (Inconsistency) |
26.9 |
95% CI for I2 |
18.84-91.6 |
Table 3 Significant heterogeneity and disagreement was found between studies

Figure 2 Heterogeneity and asymmetry was found and illustrated in funnel plot; Funnel plot of studies evaluating the association between occupational exposures and KCa. Dotted lines indicate 95% pseudo-confidence interval. Std Err: standard error and Logs odds ratio.
Association and risk of occupational exposure for bladder cancer distribution
To assess publication bias a funnel plot was derived (Figure 3), homogeneity among studies were founded. No bias accounted for differences in results among studies, which are not due to chance, after quantifying all factors. We found no significant heterogeneity and agreement between studied (Table 4).
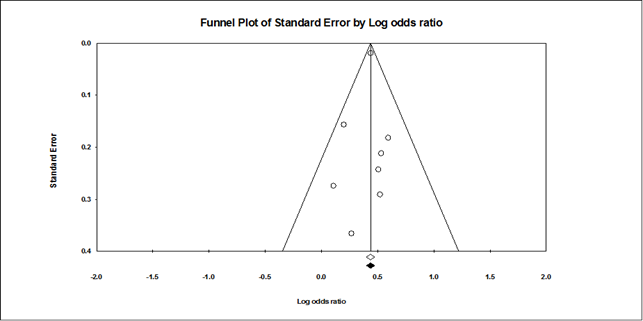
Figure 3 Homogeneity and symmetry was found and illustrated in funnel plot; Funnel plot of studies evaluating the association between occupational exposures for BCa. Dotted lines indicate 95% pseudo-confidence interval. Standard error, Log odds ratio.
Test for heterogeneity |
|
Cochran Q |
5.87 |
P |
0.215 |
I2 (Inconsistency) |
6.54 |
95% CI for I2 |
0.84-8.36 |
Table 4 No significant heterogeneity and agreement was found between studies
Categorical meta-analysis findings
Association and risk of occupational exposure for KCa distribution
Table 5 shows socio demographic distribution as mean age from all studies was 56.45±17.6 and male were majority with average percentage of 95.3% and about 42.5% were smokers. Table 6 and Figure 4 shows association and risk of occupational exposure for renal cancer distribution. Peters et al.20 (OR 1.63, 95% CI: 1.33-1.96), Saint-Jacques et al.22 (OR 1.808, 95% CI: 1.34-2.43) and Michelek et al.23 (OR 1.842, 95% CI: 1.53-2.18) showed significant risk but Michelek et al.23 (OR 0.81, 95% CI: 0.71-1.15 ), Zaitsu et al.19 (OR 1.04, 95% CI: 0.96-1.12) and Callahan et al.24 (0.95, 95% CI: 0.84-1.37 ) showed no significant risk or association. After analysis of multiple paper regarding the association between KCa and different occupational exposure we found no significant association (OR 1.04, 95% CI: 0.94-1.34).
|
Study |
N |
Age |
Sex |
Smoking |
|
|
Male |
Female |
||||
|
Michelek et al.18 |
57310 cases |
59.54±18.65 |
98.00% |
2.00% |
49.60% |
|
3316 cases |
57.48±13.65 |
81.60% |
18.40% |
74.60% |
|
|
Peters et al.20 |
712 cases |
59.0±16.32 |
100.00% |
0.00% |
16.00% |
|
Saint-Jacques et al.20 |
525 cases |
56.41±11.8 |
NA |
NA |
NA |
|
Michalek et al.23 |
59778 cases |
NA |
NA |
NA |
NA |
|
Callahan et al.24 |
1217 cases |
52.87±10.85 |
NA |
NA |
45.00% |
Table 5 Distribution of demographic data of kidney cancer studies
Z |
P |
||||
Study |
OR |
95% CI |
Forest plot |
||
Michelek et al.23 |
0.81 |
(0.71-1.15) |
1.24 |
0.215 |
|
Zaitsu et al.19 |
1.04 |
(0.96-1.12) |
1.09 |
0.271 |
|
Peters et al.20 |
1.63 |
(1.33-1.96) |
5.72 |
0.00** |
|
Saint-Jacques et al.22 |
1.808 |
(1.34-2.43) |
3.88 |
0.00** |
|
Michalek et al.18 |
1.842 |
(1.53-2.18) |
6.64 |
0.00** |
|
Callahan et al.24 |
0.95 |
(0.84-1.37) |
0.51 |
0.486 |
|
Pooled31 |
1.04 |
(0.94-1.34) |
1.78 |
0.108 |
Table 6 Association and risk of occupational exposure for KCa distribution

Figure 4 Forest plot of association occupational exposure and KCa. Box sizes reflect the weights of studies included in the meta-analysis, horizontal lines are the 95% CIs, and the summary OR is represented by the diamond. OR: odds ratio, CI: confidence interval.
Association and risk of occupational exposure for BCa distribution
Table 6 Socio demographic distribution as mean age from all studies was 59.88±8.5 and male were majority with average percentage of 83.3% and about 48.8% were smokers. Table 7 and Figure 5 shows association and risk of occupational exposure for BCa distribution; Carey et al.25 (OR 1.55, 95% CI: 1.38-1.69 ), Sciannameo et al.31 (OR 1.81, 95% CI: 1.23-2.58), Noon et al.33 (OR 1.66, 95% CI: 1.03-2.67), Golka et al.34 (OR 1.74, 95% CI: 1.12-2.53) and Hameed et al.35 (OR 1.98, 95% CI: 1.15-2.98) found and illustrated significant risk and association between occupational exposure and BCa but Turner et al.26 (OR 1.22, 95% CI: 0.86-1.65), Marant et al.28 (OR 1.30, 95% CI: 0.63-2.69) and Régis et al.30 (OR 1.11, 95% CI: 0.65-1.98) found no significant risk. After the analysis of multiple paper regarding the association between BCa and different occupational exposure we found a significant association (OR 1.54, 95% CI: 1.44-1.75) (Table 8).
|
Study |
N |
AGE |
SEX |
Smoking |
|
|
Male |
Female |
||||
|
Carey et al.25 |
111458 cases |
NA |
NA |
NA |
54.60% |
|
Turner et al.26 |
938 cases |
NA |
NA |
NA |
NA |
|
Makiko et al.27 |
10 cases |
56.0±10.25 |
NA |
NA |
NA |
|
Marant et al.28 |
7905 cases |
NA |
NA |
NA |
NA |
|
Régis et al.30 |
84 cases |
57.12±10.5 |
85.00% |
15.00% |
41.20% |
|
Sciannameo et al.31 |
893 cases |
52.87±10.85 |
NA |
NA |
45.00% |
|
Jung et al.32 |
193 cases |
NA |
NA |
NA |
NA |
|
Noon et al.33 |
17995 cases |
NA |
NA |
NA |
NA |
|
Golka et al.34 |
400 cases |
65.88±9.21 |
75.00% |
25.00% |
NA |
|
Hameed et al.35 |
100 cases |
63.74±8.65 |
100.00% |
0.00% |
60.00% |
Table 7 Distribution of demographic data of bladder cancer studies

Figure 5 Forest plot of association occupational exposure and bladder cancer. Box sizes reflect the weights of studies included in the meta-analysis, horizontal lines are the 95% CIs, and the summary OR is represented by the diamond. OR: odds ratio, CI: confidence interval.
|
Study |
OR |
95% CI |
Z |
P |
Forest plot |
|
Carey et al.25 |
1.55 |
(1.38-1.69) |
22.32 |
0.00** |

|
|
Turner et al.26 |
1.22 |
(0.86-1.65) |
1.25 |
0.251 |
|
|
Makiko et al.27 |
NA |
NA |
NA |
NA |
|
|
Marant et al.28 |
1.3 |
(0.63-2.69) |
0.73 |
0.461 |
|
|
Régis et al.30 |
1.11 |
(0.65-1.98) |
0.39 |
0.68 |
|
|
Sciannameo et al.31 |
1.81 |
(1.23-2.58) |
3.22 |
0.001** |
|
|
Jung et al.32 |
NA |
NA |
NA |
NA |
|
|
Noon et al.33 |
1.66 |
(1.03-2.67) |
2.11 |
0.037* |
|
|
Golka et al.34 |
1.74 |
(1.12-2.53) |
2.51 |
0.012* |
|
|
Hameed et al.35 |
1.98 |
(1.15-2.98) |
2.88 |
0.009* |
|
|
Pooled31 |
1.54 |
(1.44-1.75) |
23.3 |
0.00** |
Table 8 Association and risk of occupational exposure for bladder cancer distribution
This study provides a complete systematic review of the literature and quantitative estimates of the associations between occupational exposure and the risk of KCa and BCa in population-based observational studies that were published between January 2018 and May 2019. The results of our meta-analysis revealed no significant association between occupational exposure and KCa (OR 1.04, 95% CI: 0.94-1.34), whereas there was a positive association between occupational exposure and BCa (OR 1.54, 95% CI: 1.44-1.75). Wielders are predisposed to KCa because of the association between KCa and exposure to iron and nickel present in wielding fumes.17 Occupational exposures to potential carcinogens can be prevented through quality control, health and safety surveillance in work places. The association between occupational lead exposure and KCa risk have not been proven to be significant by previous case control studies.24 Exposure to a high level of asbestos is associated with KCa among Canadian industrial workers.20 Individuals working in petroleum industries are predisposed to KCa because there is a possible association between gasoline, diesel exposure and KCa.21 The relationship between occupational risk of direct exposure to aromatic amine and cancer was not significant but higher risks were found for chemical, rubber, dyers and printer workers.1 A significantly elevated risk of KCa was found among men in higher occupational class most especially in blue‐collar industries (OR, 1.61; 95% CI, 1.34‐1.93) which might be linked to job stress among Japanese professionals.19
One of the major occupational risk factors for BCa is the exposure to aromatic amines.37,38 Mathieu et al.39 found a significant higher risk of BCa among workers in a rubber production plant according to the results of their systematic review and meta-analysis ((SRR 1.36; 95% CI: 1.18, 1.57). According to Carey et al.25 cigarette smoking is a notable risk factor for BCa based on the outcome of the Nordic Occupational Cancer study, smoking cessation should be encouraged by employers. Golka et al.34 discovered a higher risk of BCa among former hard coal miners in a former area of coal, iron and steel industries in Dortmund, Germany, 70% of BCa cases were attributed to glutathione S-transferase M1 (GSTM1) negative. From the results of a meta-analysis conducted by Fang et al.40 based on 506 case-control studies GSTM1 was associated with a significantly increased risk in cancer especially in smokers (OR=1.17; 95%CI=1.14–1.21). Indicating that smoking increases the production of oxidative stress, especially in people carrying GSTM1 null genotype. These individuals are more prone to gene damage and hence increases the risk of cancer.
According to Koutros et al.41 There was no significant association between occupational exposure to fumigants or fungicides and BCa, with the exception of a positive association among smokers using carbon tetrachloride/carbon disulfide, which was based on only three exposed cases whereas Zhen et al.42 conducted quantitative analysis in order to evaluate the relationship between pesticide exposure and the risk of BCa by summarizing the results of published case-control and cohort studies, they observed that pesticide exposure was associated with an increased risk of BCa (OR=.649, 95% CI 1.223-2.223), in subgroup analysis, they observed that pesticide exposure is a significant risk factor for BCa in America (OR=1.741, 95% CI 1.270-2.388), the same result were observed in both case-control group and cohort group (OR=2.075, 95% CI 1.183-3.638, OR1.146, 95% CI 1.074-1.223, respectively).
In order to minimize the risk of KCa and BCa in working places most especially in hydrocarbon Industries, there is need for industries to design a health, safety and quality control protocols in order to achieve the standard goal of regulating the amount of carcinogenic materials utilized for production purposes and also by modelling the production processes to reduce the release of carcinogens into the environment.43 Industries should also endeavor to provide health and safety information for workers as well as setting up smoking cessation interventions at working place. Future studies are required to investigate and address the relationship between occupational exposures and risk of KCa and BCa, most especially in Hydrocarbon and Metallurgical industries. There is need for more policies and strategies to mitigate the effects of industrial chemicals on health of workers in order to minimize the risk of cancer. Moreover a global epidemiological surveillance system and database should be set up with aim of registering and analyzing reported occupational risk of KCa and BCa.
Limitation
There are some limitations in the present study. The gender effects of exposure to potential occupational carcinogens could not be analyzed in the present study, because majority of the selected studies did not report on gender effects. Moreover, there was no toxicological analyses in most of the studies.
From the result of our qualitative and quantitative analysis there was no significant association between occupational exposure and KCa, whereas there was a significant association between occupational exposure and BCa.
None.
The author declares there is no conflict of interest.

©2019 Deb, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.