eISSN: 2378-3176


Case Report Volume 11 Issue 3
1Dr.Soliman Fakeeh Hospital, Jeddah, SA
2Urology&Nephrology Center, Mansoura University, EGYPT
3Fakeeh College of Medical sciences, Jeddah, SA
Correspondence: Ahmed Akl, Urology & Nephrology Center Mansoura, Egypt, Tel +96 655 9321074
Received: September 10, 2023 | Published: September 26, 2023
Citation: Ahmed A, Maged Mazen F. Long term effects of SARS-Cov2 associated minimal change glomerulonephritis: a case report and review of literature. Urol Nephrol Open Access J. 2023;11(3):85-87. DOI: 10.15406/unoaj.2023.11.00338
Background: Due to the outbreak of SARS-COV2 in December 2019, an international COVID19 disease curfew has been imposed. COVID-19's persistent clinical symptoms harmed the respiratory system. Patients' sporadic renal symptoms may be related to viral load, immunological response, or medicines utilized.
Case report: A 37-year-old lady suffering from hypothyroidism. The patient presented to the nephrology clinic with lingering loss of smell and taste after a COVID-19 infection 6 months prior, as well as a one-week history of bilateral lower limb swelling and puffy eye lids. The urine albumin/creatinine ratio was 2786 mg/g, the total serum cholesterol was 528 mg/dl, the LDL was 423.4 mg/dl, and the triglyceride was 174.5 mg/dl, with a 24-hour urine protein collection returning 4912 mg/day. Complement 3 (C3), complement 4 (C4), and erythrocytes sedimentation rate (E.S.R) were all within normal limits, indicating that post-streptococcal glomerulonephritis, membranoproliferative glomerulonephritis, and systemic lupus erythematosus (SLE) were improbable, as complement levels are normally lowered in these disorders. Light microscopy indicated intact glomeruli, negative immunofluorescence, effacement of podocyte foot processes, and no viral particles after a renal biopsy. The condition was diagnosed as a minimal change in glomerulonephritis. The patient was started on Prednisolone 60 mg orally once daily, as well as Ramipril 5 mg orally once daily, and there was a good response to therapy after one month. The albumin/creatinine ratio decreased from 2786.56 to 5.69 mg/g, urine microalbumin fell from 7278 to 11 mg/L, total cholesterol decreased to 215.4, and urine protein decreased from 4912 to 91.8 mg/day. The oral steroids were gradually reduced in dosage. Three weeks later, all lab work was performed and showed remarkable improvement. The 24- hour urine protein content was found to be 82.6 mg/day, with an albumin/creatinine ratio of 4.44 mg/g.
Conclusion: We present a case of post-COVID-19 minor change glomerulonephritis that responded entirely to steroids and was free of sequelae for two years.
Keywords: SARS-COV2, covid-19, nephrotic syndrome, glomerulonephritis
SLE, systemic lupus erythematosus; ESR, erythrocytes sedimentation rate; C3, complement 3; C4, complement 4; MCD, minimal change glomerulonephritis; FSGS, focal and segmental glomerulosclerosis; ACE2, angiotensin-converting enzyme 2
Although the advent of nephrotic syndrome in COVID-19 is uncommon, it has been documented in patients who have not been followed for a long time.1–3 In children, the most prevalent cause of nephrotic syndrome is minimal change glomerulonephritis (MCD), whereas in adults, diseases such as focal and segmental glomerulosclerosis (FSGS) predominate.4,5 To evaluate a therapeutic option and prognosis, it is crucial to distinguish MCD from FSGS; however, this is not always an easy task in clinical practice because early FSGS can simply present with diffuse foot process effacement. COVID-19 has also been linked to several occurrences of collapsing glomerulopathy in patients.3,6 This shows that SARS-CoV-2 infection could result in severe podocytopathy in susceptible individuals.3
A 37-year-old female patient with hypothyroidism on 50 mcg levothyroxine once a day, a 6-month history of COVID-19 infection with residual loss of smell and taste, and a 1-week history of bilateral lower limb edema and puffiness of the eyes lids. When the patient's TSH level was discovered to be elevated, she was referred to an endocrinology clinic to have her levothyroxine maintenance dose modified. Urine albumin/creatinine ratio (2786 mg/g), total cholesterol 528 mg/dl, LDL 423.4 mg/dl, and triglyceride 174.5 mg/dl were all elevated. Additional confirmatory testing was carried out since nephrotic syndrome was highly suspected. A 24-hour urine protein collection revealed 4912 mg of protein excretion each day. Complement 3 (C3), complement 4 (C4), and erythrocyte sedimentation rate (E.S.R) were all within normal limits, indicating that post-streptococcal glomerulonephritis, membranoproliferative glomerulonephritis, and systemic lupus erythematosus (SLE) are unlikely, as complement levels are normally lowered in these disorders. A renal biopsy was performed, which revealed normal glomeruli by light microscopy Figure 1A, negative immunofluorescence, effacement of podocytes foot processes, and no viral particles by electron microscopy Figure 1B, confirming minimal change glomerulonephritis. The therapy with man-made corticosteroid (Prednisolone) 60 mg and Angiotensin- converting enzyme inhibitor (Ramipril) 5 mg once daily was effective in one month. The albumin/creatinine ratio fell from 2786.56 to 5.69 mg/g, urine microalbumin fell from 7278 to 11 mg/L, and total cholesterol fell from 528.3 to 215.4 mg/dl. The 24-hour urine protein measurement fell from 4912 to 91.8 mg/day. Laboratory testing demonstrated considerable improvement after three weeks of gradually tapering off oral steroids. The protein level in the urine was 82.6 mg/day, the microalbumin level was 7.6 mg/L, the albumin/creatinine ratio was 4.44 mg/g, and the protein in urine analysis was negative.
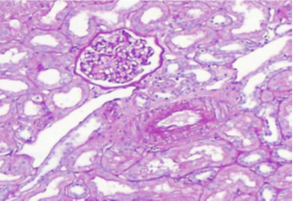
Figure 1A Light microscopy: The biopsy sample consists contain up to 21 glomeruli, 1 of them globally sclerosed. Glomerular basement membranes do not show thickening, spikes or vacuoles where tangentially cut. Adhesions, segmental sclerosis, hyalinosis, fibrin, necrosis or endocapillary proliferation or crescents are not seen. Tubular atrophy and interstitial scarring affect less than 5% of the cortex. Proximal tubular epithelium shows cytoplasmic vacuolation. Interstitium is free of inflammation. Seven interlobular arteries are included that show up to mild fibrointimal thickening. There is no arteriolosclerosis. Immunofluorescence Microscopy: sections were stained for C1q, C3, IgG, IgM, IgA, Kappa light chain & Lambda light chains, Fibrinogen and Albumin. All immunostains were negative.
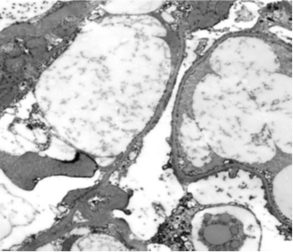
Figure 1B Electron microscopy: Two glomeruli were available for assessment. The podocytes show cytoplasmic vacuolation & 90% effacement of the podocyte foot processes. Mesangial matrix is mildly increased without electron dense deposits. Occasional endothelial cells are swollen but their fenestrations are retained. Tubuloreticular inclusions are not detected. The tubular epithelial cells show cytoplasmic electron dense and clear droplets representing phagosomes with reabsorbed protein and lipid material. Small arteries are not sampled.
The involvement of SARS-CoV-2 in renal tubules and glomeruli is substantiated by postmortem investigations of fatal cases of COVID-19. Electron microscopy showed coronavirus-like particles with characteristic spikes, while in situ hybridization and indirect immunofluorescence identified SARS-CoV-2 viral RNA and protein.7,8 During the fusion process, this virus attaches to the transmembrane protein angiotensin-converting enzyme 2 (ACE2).9 ACE2 is found in podocytes, the parietal epithelium of the Bowman capsule, and the proximal tubules of the kidney.9 MCD has been reported following the COVID-19 immunization, although there is no evidence of a link with COVID-19 disease in the literature. The rapid response to glucocorticoids in proteinuria reduction may favor MCD-type podocyte damage. Although the specific mechanism of MCD is unknown, there may be a pathogenic link between some viral infections and the subsequent T-lymphocyte activation with cytokine production, which may contribute to its pathogenesis.10 Respiratory infections, especially SARS-CoV-2, have been linked to a return of proteinuria in certain patients with nephrotic syndrome in remission.11,12 The regular use of glucocorticoids in COVID-19 treatment regimens may be to blame for obscuring diagnostic MCD connection with COVID-19 disease Table 1.
|
Time |
7/1/2021 |
20/02/2021 |
12/03/2021 |
12/03/2021 |
23/05/2021 |
30/06/2021 |
22/08/2021 |
22/01/2022 |
17/04/2023 |
|
Serum Albumin (g/dl) |
2.16 |
3.83 |
3.8 |
3.8 |
3.8 |
4.23 |
|||
|
Serum creatinine (mg/dl) |
0.73 |
0.65 |
0.72 |
0.68 |
0.68 |
0.5 |
0.51 |
0.56 |
0.58 |
|
Albumin/creatinine ratio (mg/g) |
2786.56 |
5.69 |
9.73 |
4.44 |
5.24 |
4.82 |
. |
7.45 |
|
|
Total cholesterol (mg/dl) |
528.3 |
215.4 |
186.5 |
160.5 |
194.5 |
211 |
172 |
244.2 |
|
|
LDL (mg/dl) |
423.4 |
94.6 |
141.6 |
83.1 |
162.4 |
87.8 |
|||
|
Triglycerides (mg/dl) |
174.5 |
87.5 |
95.4 |
75.7 |
70.6 |
70.9 |
58.9 |
||
|
Urine MicroAlbuminemia (mg/L) |
7278.5 |
11 |
30.9 |
7.6 |
13.4 |
9 |
17.7 |
||
|
24hour urine protein collection (mg/day) |
4912 |
91.8 |
66 |
82.6 |
43.5 |
102.9 |
57 |
63 |
43.2 |
|
HBA1C |
5.29 |
||||||||
|
ALT (mg/dl) |
7.1 |
Table 1 Follow up of renal function, lipid profile and proteinuria
However, immunohistochemistry could not confirm the presence of SARS-CoV-2 in kidney parenchyma, and electron microscopy revealed no virus-like particles. Our failure to demonstrate direct viral infection in podocytes is consistent with earlier findings that postulate cytokine milieu in response to SARS-CoV-2 infection, rather than direct viral involvement in podocytes, as a cause of COVID-19-associated nephropathy.3,6 Tubule-reticular inclusions can be detected in viral or immune-mediated podocytopathies and are thought to be interferon footprints because they are generated by both endogenous and exogenous interferon.13 Analyses of COVID-19 patients show an increase in plasma cytokines, including interferon, as well as stimulation of interferon-stimulated genes in the respiratory system.14,15 Collapsing glomerulopathy, which is characterized by podocyte hyperplasia and hypertrophy, is the most severe form of podocytopathy and one of the FSGS variations. This collapsing glomerulopathy is the histologic characteristic of HIV-associated nephropathy, which is most common in people who have the high-risk APOL1 allele.16,17 Several examples of collapsing glomerulopathy caused by COVID-19 have also been documented in nontransplant patients with a high-risk Apolipoprotein L1 (APOL1) genotype.3,6 This shows that SARS-CoV-2 infection could cause severe podocytopathy in people who carry the APOL1 risk allele.3 Clearly, we had podocyte vacuolization, which could indicate podocyte damage.
We present a case of post-COVID-19 minor change glomerulonephritis that responded entirely to steroids and was free of sequelae for two years.
None.
The authors declares that there is no conflict of interest.

©2023 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.