eISSN: 2378-3176


Case Report Volume 2 Issue 5
1Department of Nephrology, State University of Medicine and Dentistry, Russia
2Department of Pathology, National Centre of Clinical Morphology, Russia
Correspondence: Elena V Zakharova, City Clinical Hospital n.a. S.P. Botkin, 115284, 2-nd Botkinsky proezd, 5, Moscow, Russia, Tel 7 499 728 8291
Received: November 27, 2015 | Published: December 23, 2015
Citation: Zakharova EV, Vorobjova OA. Immunoglobulin g4-related disease, presented with retroperitoneal fibrosis and monoclonal gammopathy: case reportand mini review.Urol Nephrol Open Access J. 2015;2(5):168-173. DOI: 10.15406/unoaj.2015.02.00058
Immunoglobulin G4-related disease (IgG4-RD) is a new entity, which comprise a group of conditions, sharing common clinical, serologic and pathologic features-mainly tumor-like swelling of involved organs, lymphoplasmacytic tissue in filtration with the predominance of IgG4 positive plasma cells and CD4 positive T lymphocytes, modest tissue eosinophilia, and so-called “storiform” fibrosis with cartwheel appearance of the arranged fibroblasts and inflammatory cells. The pathogenesis of IgG4-RD is not fully understood, and the pathogenic role of IgG4 is still under discussion. The pathology process may involve pancreas, bile ducts, gallbladder, liver, thyroid, salivary and lacrimal glands, orbits, lungs, mediastinum, pericardium, aortae and arteries, kidneys, prostate and testes, breast, lymph nodes, skin, hypophysis etc., but one of the most common manifestations is retroperitoneal fibrosis. Several case series data showed that IgG4-RD is responsible for a majority of cases of retroperitoneal fibrosis, previously regarded as “idiopathic”. The diagnosis of IgG4-RD requires pathology data; lymphoplasmacytic tissue infiltration with mainly IgG4+ plasma cells and lymphocytes is confirmatory. Serum IgG4 levels should be measured and isolated IgG4 elevation is a significant aid in diagnosis, although it is not diagnostic. Elevated IgG4 in patients with IgG4-RD were shown to be usually polyclonal First-line treatment for symptomatic IgG4-RD is prednisone. Here we present a pathology proven case of IgG4-RD, manifested as retroperitoneal fibrosis and monoclonal gammopathy, successfully treated with corticosteroids.
Keywords: immunoglobulin g4, lymphoplasmacytic infiltration, retroperitoneal fibrosis, monoclonal secretion, prednisone
ANCA, anti-neutrophil cytoplasm antibodies; BP, blood pressure; C, complement; CD, classification determinant; CRP, c-reactive protein; CT, computer tomography; DNA, deoxyribonucleic acid; ECG, electrocardiogram; ECHO-CG, echocardiography; ESR, erythrocytes sedimentation rate; Hb, hemoglobin; HBs Ag, hepatitis b serum antigen; HCV, hepatitis c virus; HEENT, head, eyes, ears, nose, throat; HIV, human immunodeficiency virus; HPF, high power field; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; La/SS-B, sjögren’s syndrome related antigen B; Plt, platelets; PTH, parathyroid hormone; RBC, red blood cells; RF, rheumatoid factor; Ro/SS-A, sjögren’s syndrome related antigen A; RPR-test, rapid plasma regain test; RR, respiratory rate; SG, specific gravity; T3, tri-iodothyronine; T4, thyroxin; TSH, thyroid stimulating hormone; WBC, white blood cells
Immunoglobulin G4-related disease, previously also described as IgG4-syndrome, IgG4-related systemic sclerosing disease, IgG4 autoimmune disease, systemic IgG4-related plasmacytic syndrome, IgG4-positive multiorgan lymphoproliferative syndrome, multifocal idiopathic fibrosclerosis etc, is a new entity, which comprise a group of conditions, sharing common clinical, serologic and pathologic features. Main changes, characteristic for IgG4-RD are tumor-like swelling of involved organs, lymphoplasmacytic tissue infiltration with the predominance of IgG4 positive plasma cells and CD4 positive T lymphocytes, modest tissue eosinophilia, and so-called “storiform” fibrosis with cartwheel appearance of the arranged fibroblasts and inflammatory cells; 60-70% of patients demonstrate also elevated IgG4 serum concentrations.1‒9 IgG4-RD is regarded as a fibro inflammatory condition, which pathogenesis is not fully understood, and the pathogenic role of IgG4 is still under discussion. IgG4 is a unique bivalent blocking immunoglobulin; it does not fix complement and does not bind efficiently to Fcγ receptors, suggesting its role as an immuno modulator, rather than inflammatory antibody. Recent insights into the pathophysiology of IgG4-RD also suggest that one of the major drivers of the disease appear to be plasmablasts. Oligoclonal expanded plasmablasts with extensive somatic hyper mutation were found to be increased in IgG4-RD patients with active disease.9‒12
The process may involve pancreas, bile ducts, gallbladder, liver, thyroid, salivary and lacrimal glands, orbits, lungs, mediastinum, pericardium, aortae and arteries, retroperitoneum, kidneys, prostate and testes, breast, lymph nodes, skin, hypophysis etc. Clinical manifestations, actually regarded in the setting IgG4-RD, include broad variety of conditions.3‒5,13‒30
Thus, the diagnosis of IgG4-RD should be at least considered in patients with one or more of the above listed characteristic patterns of organ/tissue involvement. Given the most often involved target organs, the specific diagnostic search is strictly needed in the patients with:
Retroperitoneal fibrosis is one of the most common manifestations of IgG4-RD. Several case series data showed that IgG4-RD is responsible for a majority of cases of retroperitoneal fibrosis, previously regarded as “idiopathic”. IgG4-related retroperitoneal fibrosis (RPF) involves particularly the infrarenal aorta, iliac arteries and ureters, causing obstructive uropathy, and may present as isolated RPF, or in some cases may be accompanied by IgG4-related pancreatitis, sialadenitis, lymph node sclerosis, hypophysitis, mediastinal periaortitis, sclerosing mesenteritis, sclerosing mediastinitis, and multifocal fibrosclerosis.31‒39 On the other hand, RPF may present as a “secondary” form, major causes of which are.40
Given multiple conditions, which may lead to RPF, differential diagnostics demand screening for above mentioned diseases and factors. Confirmation of the diagnosis of IgG4-RD requires pathology data, such as lymphoplasmacytic tissue infiltration of mainly IgG4 positive plasma cells and lymphocytes, accompanied by fibrosis that has “storiform” features, and often by obliterative phlebitis and modest tissue eosinophilia. Serum IgG4 levels should be measured, and isolated IgG4 elevation is a significant aid in diagnosis, although it is not diagnostic.41,42
Treatment for IgG4-RD is not yet established, International consensus statement tells that prednisone should be the first-line treatment in patients with symptomatic IgG4-RD, unless contraindicated, and recent studies confirm benefit of corticosteroids in patients with IgG4-related kidney disease. There are also increasing data in favour of rituximab usage for IgG4-RD, mostly in patients not responding to prednisone.43‒46
Caucasian lady 57years old, admitted to nephrology unit May 5 2015
Main complains
General weakness, moderate back pain, decreased appetite, and weight loss.
Previous medical history
Duodenum ulcer for manyyears, last time symptomatic in 2014 and treated with omeprazole; uterine fibroid (uterine extirpation in 2004); inguinal hernia (hernioplasty in 2011).
History of current disease
January 2015 she developed unexplained weakness, thirst and weight loss. Outpatient work-up found anemia (Hb 8.8g/dL) and decreased renal function (serum creatinine 240µmol/L). Abdomen ultrasound showed bilateral hydronephrosis, gastroscopy found nothing but duodenum cicatricle deformity, colonoscopy findings were unremarkable.
March 2015 she was admitted to urology clinic with her serum creatinine raised up to 450µmol/L, CT confirmed bilateral ureterohydronephrosis, and found right kidney enlargement and retroperitoneal mass with bilateral ureteral compression (Figure 1). She was diagnosed with retroperitoneal fibrosis, underwent right kidney percutaneous nephrostomy and left ureteral stenting, her creatinine lowered to 241µmol/L and she was referred to our clinic for further evaluation.
At admission
Conscious, alert, oriented, slightly depressed. Body temperature 36.9°C, RR 18 per minute, pulse regular 84 per minute, BP 140/80mm Hg. Pale, undernourished. No peripheral oedema, neither palpable peripheral lymph nodes.
Joints: No swelling, movements not restricted. HEENT and neck otherwise normal.
Lungs: No dullness to percussion, any rhonchi, wheezes or rubs.
Heart: Regular rhythm, no murmur. Abdomen soft, non-tender, bowel sounds normal. Liver +1cm below rib arch, non-painful, spleen and kidneys not felt. Right kidney urine drainage functioning well, urine output via drainage 1000 mL/day, spontaneous urine output 900mL/day, urine normally colored, slightly opaque.
Work up
Total blood count: Hb 11.0g/L, WBC 8.2x109/L with normal formula, Plt 166x109/L, ESR 35mm/h.
Blood chemistry: creatinine 225µmol/L, urea 20.1mmol/L, uric acid 560µmol/L, total protein 82g/L, serum iron 6.7µmol/L; electrolytes, glucose, albumin, cholesterol, bilirubin, liver enzymes - within normal range.
Urinalysis: SG 1012, protein 0.4g/L - 0.69g/day, RBC 3-5, WBC >200hpf.
Urine culture: Klebsiella species 1x108/mL.
Infections screening: RPR-test for Тreponema Pallidum, HBsAg, anti-HCV and anti-HIV-antibodies negative, tuberculin skin test-all negative.
Autoimmune screening: RF, anti-DNA antibodies, anti-Ro/SS-A, anti-La/SS-B antibodies, C3, C4, pANCA, cANCA, IgG, IgA, IgM and CRP-all within normal range, cryoglobulines negative.
Hormones: T3, T4, TSH and PTH within normal range.
Serum protein high resolution electrophoresis: Mild IgG-κappa monoclonal secretion, confirmed by immunofixation and immunophenotyping.
Urine protein electrophoresis: Mild tubular proteinuria.
IgG subclasses: Total IgG 12.7g/L, IgG1 60.5%, IgG2 34.0%, IgG3 3.2%, IgG4 2.3% (all within normal range).
ECG; chest and skeletal X-Ray; ECHO-CG and aortae; pelvis and peripheral lymph nodes ultrasound: Unremarkable.
Abdomen and kidneys ultrasound: Gallbladder lithiasis, liver and pancreas otherwise normal, mild splenomegaly (140x65mm).
Kidneys: Right 116x47, left 101x45mm, with hyperechoic parenchyma 17-20mm.
Mild bilateral collecting system dilatation: Right pelvis 15mm, calices 12mm, left pelvis 14mm, calices 8mm. Urine drainage in the right pelvis, stent in the left ureter. No visible retroperitoneal lymph nodes. Stringy hypoechoic retroperitoneal masses, 6x12x15 mm, surrounding aorta and both ureters.
Thyroid gland ultrasound: Right lobe 50х17х17mm, left lobe 45х17х15mm, isthmus 4mm. Echogenicity uneven, texture irregular, multiple nodules maximum 11х6mm. Regional lymph nodes enlarged up to 12-14mm with normal structure.
Bone marrow biopsy: No changes, specific for lymph proliferative disease found.
Diagnostic considerations and further work-up
At that point patient with retroperitoneal fibrosis was evaluated for aortic aneurism, Riedel’s thyroiditis, ANCA-associated vasculitis, systemic lupus erythematous, cryoglobulinemia, rheumatoid arthritis, infections and solid tumors, and no proof for these disorders was found. On the other hand, presence of low-grade paraproteinemia and splenomegaly was suggestive for extra-organ lymphoma and demanded pathology evaluation of retroperitoneal masses. Ultrasound-guided biopsy was technically unachievable. Patient was treated with antibiotics for urinary tract infection, then urine drainage was removed and right ureter stent placed instead, after that she was referred to the surgical unit for endoscopic biopsy of retroperitoneal mass.
Biopsy of retroperitoneal mass: pathology evaluation (Figures 2‒4) by light microscopy sections of formalin fixed paraffin embedded tissue, stained with periodic acid-Schiff and Haematoxylin-Eosin, represented mostly coarse fibrous tissue with prominent desmoplastic changes, and focal lymphoid infiltration with substantial admixture of plasma cells. Fine focal lymphocyte and plasma cell aggregates were found also per vascular in the depth of fibrous tissue. Immunohistochemistry on formalin fixed paraffin embedded tissue with immuno peroxidase staining for IgG4, CD138, CD3 and CD20 showed diffuse (80%) prominent expression of CD20+, diffuse multifocal (40%) prominent expression of CD138+, and focal (<20%) moderate expression of CD3+. Vast majority of CD138 positive cells showed also diffuse moderate expression of IgG4 (up to 15-20 IgG4 positive plasma cells per field with magnification x400).
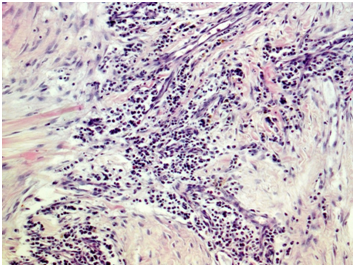
Figure 2 Light microscopy showing patchy perivascular, predominantly plasma cell infiltration in the fields of irregular fibrosis. (H&E, x 100.).
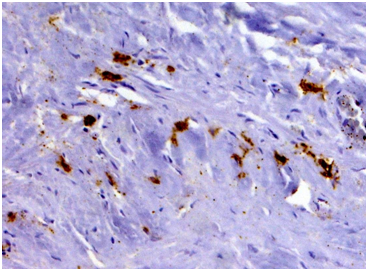
Figure 3 Immunohistochemistry revealing patchy clusters of CD138-positive plasma cells in the fields of coarse fibrosis. (Immunoperoxidase, х 100.).
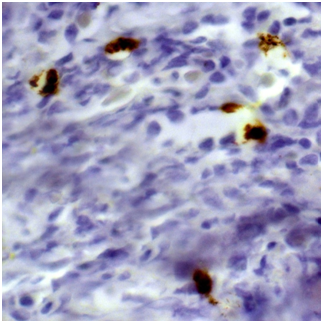
Figure 4 Immunohistochemistry showing seven IgG4-positive plasma cells per hpf (Immunoperoxidase, х 400.).
Pathology conclusion: IgG4-associated retroperitoneal damage with massive desmoplastic fibrosis and focal, mainly B-cell lymphoid infiltration with plasma cell transformation (CD20+, CD138+, IgG4+).
Final diagnosis and further treatment: Patient was diagnosed with IgG4-related retroperitoneal fibrosis and started on prednisone 40mg/day.
At last evaluation
2months later (October 2015) she is doing well, no complains, gained 4 kilos weight, BP 120/80mm Hg, urine output 1500ml/day, and physical examination unremarkable. Hb 144g/L, WBC 6.6x109/L, Plt 184x109/L, ESR 4mm/h; creatinine 160µmol/L, urea 16.7mmol\L, other blood chemistry tests within normal range. Urinalysis: protein absent, RBC 30-40, WBC 10-20hpf. Abdomen ultrasound showed partial regression of previously seen retroperitoneal masses. She was retreated with antibiotics for asymptomatic urinary tract infection and advised slowly taper prednisone to 30mg/day.
Patient presented with clear retroperitoneal fibrosis and bilateral ureterohydronephrosis with impaired renal function. After the restoration of her urine passage, the question of the cause for RPF was raised. As a first step, screening for the diseases and conditions, which may lead to the “secondary” RPF was carefully performed. Patient denied usage of any medications but omeprazole; she also denied traumas, exposure to radiation and surgery, except uterine extirpation and inguinal hernioplasty. The results routine labs, infectious and autoimmune screening, thyroid and parathyroid hormones tests, chest X-Ray, gastro copy and colonoscopy, ECHO-CG, aortae, neck, abdomen, kidneys, pelvis and peripheral lymph nodes ultrasound allowed us to rule out autoimmune systemic and inflammatory diseases, viral hepatitis, tuberculosis and solid tumors. The only findings, beyond retroperitoneal masses, were thyroid nodules, gallbladder lithiasis, mild splenomegaly and low-grade IgG-kappa paraproteinemia.
That turned us to the differential diagnostics between extra organ B-cell lymphoproliferative disorder and IgG-related disease. Bone marrow biopsy did not reveal any proof for lymphoproliferative disease, and IgG4 serum level was found to be normal, thus none of these two conditions were not confirmed neither excluded and we need pathology confirmation of the diagnosis. Endoscopic retroperitoneal mass biopsy provided the clue to the diagnosis. Pathology examination with immunohistochemistry found massive desmoplastic fibrosis and focal, mainly B-cell lymphoid infiltration with plasma cell transformation (CD20+, CD138+, IgG4+), which is characteristic for IgG4-RD.41,42
Based on these findings, IgG4-related retroperitoneal fibrosis was diagnosed. Taking into consideration that elevated serum levels of IgG4 are observed only in 60-70% of patients with IgG4-RD and not necessarily needed to confirm the diagnosis.7,41,42 normal level of IgG4 don’t rule out this diagnosis. We believe that RPF in this particular case is the solely one manifestation of IgG4-RD, as we did not find any data in favor of aortic aneurism, pericarditis, Riedel’s thyroiditis and pancreatitis or any other typical for IgG-RD organ involvement. Gallbladder lithiasis without signs of holecystitis or cholangitis is probably comorbid condition rather than IgG4-RD manifestation, as well as small thyroid nodules not accompanied by thyroid dysfunction. Patient was treated with prednisone according to International consensus statement,41 which resulted in the resolution of clinical symptoms and partial regress of retroperitoneal masses.
The most controversial issue is the significance of mild IgG-kappa paraproteinemia. Elevated IgG4 in patients with IgG4-RD were shown to be polyclonal.5,9 On the other hand, patients with IgG4-RD have a 3.5 times higher risk of cancer than the general population. Reported cases are predominantly extranodal marginal zone lymphomas (MALT lymphomas), which either may arise in the background of a pre-existing non-clonal IgG4-related chronic sclerosing lesion, or have evidence of plasmacytic differentiation with IgG4-positive monoclonal plasma cells.47‒50 Few cases of the association of IgG4-RD, POEMS and Castleman disease also are reported.51‒53 Several studies of subclass distribution in IgG monoclonal gammopathy of undetermined significance and myeloma, performed back in the 1980s, showed that IgG4 myeloma represented 2-8% of IgG plasma cell neoplasms.54‒57
The recent study of more that 150 patients with IgG plasma cell myeloma found that IgG4 myeloma represented 4% of cases (6 out of 158). Remarkable, that two of six patients had a documented phase of monoclonal gammopathy of undetermined significance or smoldering myeloma before developing overt plasma cell myeloma. None of these 6 patients met criteria for IgG4-RD, but one of them had an episode of pancreatitis, another had chronic sinusitis, and two patients presented with necrotizing fasciitis. Authors postulate that IgG4 myeloma should be considered in the differential diagnosis of patients with high serum levels of IgG4.58 Another recent study found that the focal band, detected by electrophoresis in sera from patients with IgG4-RD, can be in fact polyclonal while tested by immunofixation or immunosubtraction.59 Our patient with IgG4-RD does not fulfill criteria for myeloma, but she has a monoclonal gammopathy IgG-kappa, confirmed by immunofixation and immunophenotyping. This demands follow-up and annual serum immunochemistry tests, evaluating putative progression to myeloma.
Olga Vinogradova, Tatyana Makarova, Ivan Lebedinsky, Andrey Evsikov, Dmitry Kolbasov and Natalya Ivanova.
Author declares that there is no conflict of interest.

©2015 Zakharova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.