eISSN: 2378-3176


Case Report Volume 5 Issue 4
0Wilkes University, USA
0Nephrology Hypertension Renal transplant and Renal Therapy, USA
0Rutgers New Jersey Medical School, USA
Correspondence: Alexander M Swan, AA Prof of Medicine, Rutgers New Jersey Medical School, 185 S Orange Ave, Newark, NJ 07103, President, Garden State Kidney Center, 345 Main Street, Woodbridge, NJ 07095, USA, Tel 732-750-5555, Fax 732-750-5550
Received: November 10, 2017 | Published: October 26, 2017
Citation: Swan AM, Fried NJ, Lee C, Aung TM, Aye ZN, et al. (2017) Extensive Skin Necrosis Ulcers and Systemic Vascular Insufficiency Syndrome with Calciphylaxis: A Case Report. Urol Nephrol Open Access J 5(4): 00180. DOI: 10.15406/unoaj.2017.05.00180
Calciphylaxis is a part of systemic vascular calcification that is commonly seen in patients with end stage renal disease. It presents as skin ischemia and necrosis due to calcification of dermal arterioles. There is no consensus on optimal treatment and the condition is associated with a high mortality rate. Here we report a patient on peritoneal dialysis with a high calcium-phosphorus product and extensive calciphylaxis involving the extremities, back and scalp. We used a multi-interventional approach to treat this patient with a good outcome. The underlying pathology of systemic vascular calcification and insufficiency may involve other vascular beds such as the coronary and cerebral vasculature. Early detection of these conditions may improve outcomes in these patents.
Keywords: necrotic ulcer of skin, calciphylaxis, calcium-phosphorus product, vascular calcification, systemic vascular insufficiency
ESRD, end-stage renal disease; CUA, calcific uremic arterilopathy; LMWH, low molecular weight heparin
Calciphylaxis or calcific uremic arteriolopathy is a part of the continuum of systemic vascular and soft-tissue calcification that is common in end-stage renal disease (ESRD). The prevalence of vascular calcification among patients with CKD, especially those on dialysis, is reported to be more than 80%.1 Coronary artery calcification leading to coronary artery disease is the most common cause of death in dialysis patients. Calcification, fibrosis, and thrombus formation involving the dermo-hypodermic arterioles leads to a reduction in arteriolar blood flow causing the painful ischemic necrosis of Calcihylaxis.2 Chronic kidney disease-mineral bone disease and its treatment are considered to play an important role in its pathogenesis. Hyperparathyroidism, active vitamin D administration, hyperphosphatemia, and an elevated plasma calcium x phosphate product (Ca x P) have been implicated in Calcific uremic arterilopathy (CUA).
The optimal treatment for CUA is not known and has a high mortality rate. A multi-interventional strategy is likely to be more effective than any single therapy.3 Sodium thiosulfate, optimization of mineral bone parameters, bisphosphonates, pain management, wound management, antibiotic therapy, supplemented by intensified dialysis and avoidance of risk factors such as vitamin K antagonist has been advocated in the latest reports.3,4 Here we report a good outcome in a patient on peritoneal dialysis with extensive calciphylaxis without the use of sodium thiosulfate.
A 51year old African American Male presented to us with signs and symptoms of severe uremia and extensive painful ulcers over both lower extremities. He was diagnosed with end stage renal disease secondary to hypertension and peritoneal dialys is was initiated 5years ago after multiple access problems with hemodialysis. He had a past medical history of atrial fibrillation and a cerebrovascular accident 2years ago and has no residual deficits. He had a prolonged ICU stay 6months ago after resuscitation from a cardiac arrest secondary to hyperkalemia (K =8.4 mmol/L). His ICU stay was complicated by sepsis and aspiration pneumonia. He complained of excruciating pain in the lower back and both lower extremities which gradually progressed over a one month period. It was associated with painful restricted movements and numbness and tingling over the feet. On physical examination he was found to be cachectic, aggressive, combative and in severe pain. Oxygen saturation was 90% and he was hemodynamically stable. Chest auscultation showed decreased breath sounds over bilateral lung bases. Heart sounds were normal with no rubs, gallops or murmurs.
Examination of the lower extremities revealed erythematous, shiny skin on the medial surface of both lower extremities with necrotic ulcers and subcutaneous nodules. Skin was extremely tender to light touch (Figure 1&2). At admission he had microcytic hypochromic anemia (Hb=7.9g/dL) and neutrophilic leucocytosis (WBC=20.6 K/UL, 90% neutrophils). Metabolic panel showed azotemia (BUN =135, Creatinine=14.2mg/dL), symptomatic hyperkalaemia (K=6.7mmol/L with peaked T waves on EKG), metabolic acidosis (Bicarbonate =20 mmol/L) and mild hyponatremia (Na=131mmol/L). The Calcium phosphorus product was 146.3 (Corrected Calcium =11.0 mg/dL, Phosphorus =13.3mg/dL). He had secondary hyperparathyroidism (PTH=218pg/mL). Liver enzymes were elevated and severe hypoalbuminemia was noted (albumin =1.3mg/dL). Blood cultures were sterile. Chest X-ray revealed a right basal consolidation and bilateral pleural effusions.
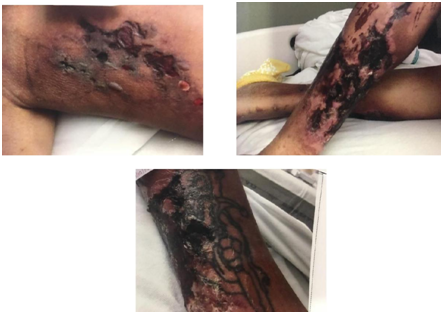
Figure 1 Necrotic ulcers over a) medial aspect of right thigh b) medial aspect of right leg and c) left Shin.
Arterial Doppler of both lower limbs showed diffuse right lower extremity peripheral arterial disease of moderate to advanced severity and left popliteal and infrapopliteal moderate peripheral arterial disease. He was managed with 12 X 2L exchanges of peritoneal dialysis with 1.25% fluid and IV antibiotics in addition to anti-hyperkalemic measures. He was started on phosphate binders Sevlemer and Sucralfate. High dose Cinacalcet therapy was started. Patient refused treatment with Sodium thiosulfate. Dual anti-platelet therapy with Aspirin and Ticagrelor was continued. Pentoxyphylline and Low Molecular weight Heparin were added. Pain was adequately managed with hydrocodone. After aggressive peritoneal dialysis and above measures he improved clinically within 1 week. The pain score and size of necrotic ulcers have significantly reduced. He is currently being followed on outpatient basis with regular peritoneal dialysis.
Our approach stresses targeting the systemic vascular calcification to treat calciphylaxis. Firstly, aggressive dialysis by means of 2hour exchanges of peritoneal dialysis decreased uremic symptoms significantly. The chronic inflammatory milieu of uremia contributes to vascular calcification and its correction prevents further propagation of the condition. A 1.5% Dextrose peritoneal dialysis solution was used. The lower osmolarity as compared to the previously used 2.5% solution prevented dehydration. Adequate hydration is vital to maintain optimal microcirculation. Additional caution was taken to prevent hypotension in order to maintain perfusion pressures in the extremities. Dual anti-platelet therapy with Aspirin and Ticagrelor was continued. Antiplatelet therapy significantly reduces the incidence of death and cardiovascular events and prevents progression of local disease in PAD patients. Combined antiplatelet therapy is more effective than aspirin alone only in patients with a history of established vascular disease.5
Pentoxifylline was also found to be beneficial in the management of this patient. Pentoxifylline, a rheologic modifier increases deformability of red blood cells and blood viscosity, decreases fibrinogen concentration, and reduces platelet adhesiveness. It has proven to be beneficial in the treatment of claudication.6 Low molecular weight heparin (LMWH) added as prophylaxis for deep vein thrombosis may have added benefits in calciphylaxis. LMWHs have proved to be superior and at least as effective as UFH in several clinical conditions where thrombosis is an important feature of arterial disease.7 However, little research has been done to investigate the potential applications of LMWH therapy for peripheral arterial pathologies. Therapy with phosphate binders and Cinacalcet helped reverse associated bone-mineral disorder and reduced the calcium-phosphorus product. Intravenous antibiotic therapy also helped reduce superadded bacterial infections and speeded the recovery process.
The mental status changes and behavioural changes seen in this patient can possibly be attributed to subtle signs of calcification in the cerebral vasculature. Other possibilities include uremia and dependence on pain medications. A growing body of evidence demonstrates atherosclerotic plaques in the circle of Willis in Alzheimer’s disease and multiple other neurodegenerative diseases. Cerebral arteriosclerosis is a part of systemic arteriosclerosis and can lead to cerebrovascular disease. Hence, early diagnosis and prevention is important. Coronary vascular disease due to coronary atherosclerosis is the leading cause of mortality in patients on dialysis. Extensive calciphylaxis is a marker of underlying systemic vascular calcification in other vasculature. Early detection and treatment of these underlying conditions helps improve outcomes in these patients.
Calciphylaxis is to be approached as a part of systemic vascular calcification. A multi-interventional strategy is required to optimally treat this condition. Coronary and cerebrovascular calcification may co-exist in these patients. It is important to screen for these conditions to diagnose and initiate appropriate treatment at the earliest to improve outcomes.
I want to thank all the authors for their valuable effort in this manuscript.
The authors declare that there are no conflicts of interest regarding the publication of this paper.
None.

©2017 Swan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.