eISSN: 2378-3176


Short Communication Volume 8 Issue 1
Specialist (A), Nephrology & Internal Medicine, Al Zahra Hospital Sharjah, United Arab Emirates
Correspondence: Sameh Elsayed, Specialist (A), Nephrology & Internal Medicine, Al Zahra Hospital Sharjah, United Arab Emirates
Received: November 16, 2019 | Published: January 20, 2020
Citation: Elsayed S. Early urological complications post kidney transplant. Urol Nephrol Open Access J. 2020;8(1):1-4. DOI: 10.15406/unoaj.2020.08.00264
renal transplantation, therapeutic approach, vascular, urological, fluid accumulation, urine leaks, retroperitoneal area, transplant area, elevated creatinine, oliguria
Renal transplantation provides superior outcomes compared to patients on dialysis. Allograft dysfunction can be managed in a time rational diagnostic and therapeutic approach and hence classified into immediate, or early (within 1-12 weeks) and late (>3-6 months) with some overlaps. Post-transplant draw backs in the early phase can be widely categorized into vascular, urological, fluid accumulation and wound recovery problems. The incidence of post-transplant surgical complications has improved over time with adapting better surgical techniques, early recognition and proper intervention.
Even with the remarkable advances in the transplantation realm, seldom cases of grafted transplants fail due to urological complications. Urological hurdles, especially urine leaks, continue to be the most prevalent surgical complication post graft transplantation. Urine leaks and fluid accumulation post-transplant is commonly due to lymphocele, hematoma, urinoma or seroma. Clinical scenario is either instant or early stages post-transplant and can be asymptomatic/or may present with pain and swelling in the transplant area, elevated creatinine, oliguria or anuria and/or signs of systemic inflammatory response.1 Urine leaks if not drained properly may provoke the development of urinomas. Additionally, urine leaking may accumulate into the retroperitoneal area, pelvis and /or in the pre-sacral and scrotal areas. These collections can squeeze the vasculature anatomy or urine outflow tract, causing graft malfunction. Add on, urine leaks are risky for wound infection, leading to peri-nephric abscesses.2,3 A systematic panel approach of investigation is adapted for establishing the diagnosis of urine leak, where the creatinine and potassium levels of the drain is compared with serum levels.4,5 Sequential ultrasound exam of the relocated graft in early post-operative stages is crucial for early etiological discovery, resolve the nature and size of the collection and exclude obstruction.6,29 Ultrasound guidance for differential diagnosis of the nature of fluid collections is challenging.
A well-defined, briskly growing, non-echoic fluent collection without septa define urinomas, while, hematoma has a multiplex echogenic look with multiple septations (Figure 1).7,8 Beside, being noninvasive technique, ultrasound maybe helpful for further evaluation of graft condition by estimating the intra-renal resistive indices.9,29 Non-contrast CT scan may be used for evaluation of nature and size of the fluid accumulation, with advantages of evaluation of graft vasculature. CT scan provides better anatomical data for proper evaluation of the etiology of hydronephrosis and defining the obstruction site. Contrast and Non-contrast-CT scan can be used according to renal graft functions and the expected etiology of Leak / obstruction and for confirming ureteric leak or necrosis.10 Isotopic scan (scintigraphy), with tracer images using MAG3 or Tc99 can define the leak, except in cases with DGF or ureteral stasis.11 Cystography using retrograde contrast may reveal bladder or anastomotic defects. Ante-grade pyelogram through percutaneous nephrostomy for realistic localization of the leak, even with DGF.12
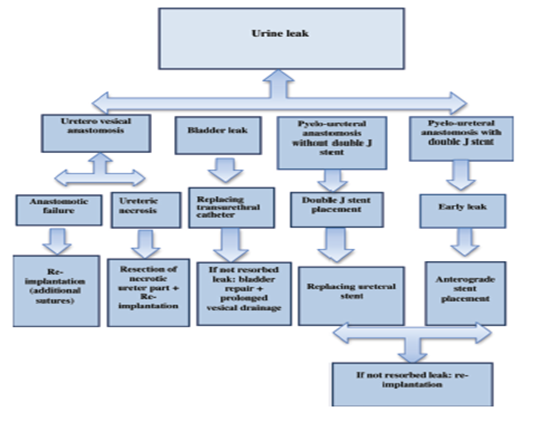
Figure 1 Algorism for diagnosis and management of urine leak Post-transplant29
Differential diagnoses
Post-operative urological complications are alerted by drain fluid color (clear, hemorrhagic, and turbid) and odor (urine like, foul).29 The sequence of events and the clinical pictures aid to clarify the differential diagnosis, as follows:
The hazards of bleeding are up to 4.9 % endanger graft malfunction or death. Post-operative hemorrhage presents with hemorrhagic drain, localized swelling and hemodynamic instability (tachycardia, low BP). Definitive diagnosis using imaging to show a hematoma .Urgent surgical intervention and exploration is indicated. Turbid drain fluid with fever, swelling, or localized graft tenderness may indicate a peri-nephric abscess, that can be affirmed with imaging and sampling of the collection for microscopy and culture.13 Lymphocele or seroma has a clear drain affirmed with imaging and comparing fluid biochemistry to serum values. The drain creatinine and potassium levels are equal to serum values, a lymphocele or seroma is most likely, lymphoceles that are not causing hydronephrosis and not associated with graft dysfunction, intervention is not indicated. while if the drain fluid creatinine and potassium are notably elevated than the serum levels, a leakage or urinoma is diagnosed.14 In our case scenario, the patients have markedly increased drainage from an indwelling drain with creatinine and potassium level disproportionately high to serum values owing to peritoneal reabsorption differentiating it from lymphocele and seroma, where serum levels would be similar.15 Retrograde or ante-grade pyelograms will demonstrate leakage of contrast. A renal isotopic scan or CT scan may show leakage of the radioisotope or the contrast. Contrast remains inside the collecting system in case of lymphocele/seroma or hematoma. A Foley’s catheter is placed immediately to help reduce intra-vesicular pressure and decompress the bladder. Further management may include placement of percutaneous nephrostomy tube, double- j ureteric stenting (if not applied intra-operative) or surgical drainge.16
Urine leak is prevalent in up to7% of kidney transplants, the clinical image depend on the site and volume of leak, decompression status of urinary tract, and graft function. High-volume leak with healthy function usually produce high drain output or external leak through the wound. Reduced urine output through the transurethral catheter and raised serum creatinine due to reabsorption, can be misdiagnosed as DGF. Nephrogram will show peri-nepheric fluid collection, showing different grades of collecting system dilatation according to obstruction.17 Patients may systemically show up by fever, hemodynamic instability, anemia, or by localized painful, swollen graft. Local signs may be minimal or obscured due to immunosuppression, so patient with high drainage is highly suspected for urinary leakage.18 Ureteric stenosis or obstruction, mostly, due to poor technical implant into the bladder, obstruction by intra-luminal blood coagulum, sloughed materials and stenosis due to fibrosis secondary to ischemia or rejection. The distal part of the ureter near to the uretero-vesical junction is the most commonly entail due to ischemia at its anatomical site, being fare from the arterial supply by the renal artery. Variable small arterial branches supply the proximal ureter from the main renal artery.27 During early post-operative period or at the onset of recovery of post ATN, diuresis in patients with delayed function. It is most commonly seen at the level of distal ureter but can occur at level of renal pelvis or mid ureteric due to the injury at time of procurement. Complications related to wound healing are commoner when using mammalian target of rapamycin (mTOR)-based immunosuppression.19
Routinely, ureteric stent is used for the first few weeks after transplantation (in most centers) for preservation against ureteric ischemia typically presenting in early weeks post-transplant or following stent removal.20 Small leak exhibit low drainage or externally through the wound, with swollen painful graft and low urine output. Renogram may reveal urinoma with/ without pelvi-calyceal dilatation and cited with nephrogram or scintigraphy. High drain levels of creatinine and potassium in comparison to serum values assure the diagnosis whether high or small volume leak. Leakage is 2ry to obstruction and relived with proper decompression by catheterization. Infection may complicate, where surgical intervention may be required.21 Intra-abdominal leaks can produce acute abdomen, shock, or ileus and always requires surgical intervention.22
Urinary tract obstruction
Urinary tract obstruction may lead to acute allograft malfunction, and may cause Leakage at any stage post transplantation but commonly early period post-operative. Compression by enlarged prostate is the main extrinsic obstruction especially in elderly males producing bladder outlet obstruction; fewer are the pressure by lymphocele, hematoma or any collection. Infrequently, calculi can produce obstructive uropathy. Clinically, a rising serum creatinine without localizing symptoms, occasionally high pressure due to obstructive uropathy may result in rupture or leakage. Ultrasound is 1st, non-invasive investigation ,will demonstrate hydronephrosis, excluding peri-ureteric collections and ensuring normal transplant perfusion while serial scan will show worsening of the hydronephrosis (as it is common post-transplant to have hydronephrosis especially early post–operative period ), so repeatedly sonographic assessment together with serial renal functions for assessment in such cases with minimal calyceal dilatation.21 Isotopic Reno-gram with the use of diuretic may help in indefinite cases. Percutaneous ante-grade pyelography is the most useful diagnostic technique, where the obstruction site is confirmed. Complicated cases may require open surgical treatment. Extrinsic compression e.g., lymphocele may require drainage procedure. Bladder outlet obstruction caused by enlarged prostate can be treated by bladder catheter and medically with drugs as tamsulosin, while advanced cases by surgical intervention.23,28
Management plan
The following diagram is showing the algorithm for management of such cases. Where early function of the graft excludes delayed graft dysfunction as a cause. According to the site, etiology and the degree of urine leak, management is either conservative or surgical according to best practice and each center experiences. Conservatively using a percutaneous nephrostomy then ante-grad stenting of the ureter, with a Folly’s catheter placement (Figure 2). As retrograde stenting of the ureter is practically difficult even with high expertise due to atypical position of the ureteric opening.25 Surgical intervention in large volume, uncontrolled urine leak with catheterization for decompression with large urinoma or necrosis of the ureter. Surgical de-necrotizing of the ureter must be proximal up to healthy ureteric tissue then re-implantation. Obstruction of folly’s catheter can be rolled out with repeated flushing with saline.26 Algorithmic approach to investigate suspected urine leak (Figure 3).

Figure 2 Algorithmic approach to investigate suspected urine leak.18

Figure 3 Management algorithm for urine leak.18
The predominance of patients received renal transplantation has a dramatic improvement in excellence of life and overall outcomes with minimal morbidity compared to dialysis patients. Post-transplant urological complications is a challenging dilemma that require participation of the transplant medical and surgical teams for proper diagnosis and management of post-transplant complications. Few patients with surgical or/and urologic complication may need an open surgical correction. Fortunately, advances in equipment and techniques allowed a lot of patients to be managed effectively with minimally invasive techniques.
None.
The author declares there is no conflict of interest.

©2020 Elsayed. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.