eISSN: 2378-3176


Research Article Volume 5 Issue 1
1Department of Human Genetics, Guru Nanak Dev University, India
2Department of Nephrology and Research, Sir Ganga Ram Hospital, India
3Aligarh Muslim university, off campus Murshidabad, India
Correspondence: Kumar Digvijay, Department of Human Genetics, Guru Nanak Dev University, Amritsar, Department of Nephrology and Research, Sir Ganga Ram Hospital, Delhi, India
Received: November 18, 2016 | Published: June 23, 2017
Citation: Chandel S, Doza B, Digvijay K (2017) Association of High Altitude Hypertension with Angiotensin Converting Enzyme (ACE) Gene Insertion/ Deletion Polymorphism. Urol Nephrol Open Access J 5(1): 00155. DOI: 10.15406/unoaj.2017.05.00155
The study included ACE gene I/D polymorphism and its association between high altitude hypertension. Genetic, biochemical, anthropometric and Physiometeric results were analyzed using statistical software. The results were non-significant for I/D polymorphism.
Objective: ACEis the major enzyme of hypertension and with most commonly reviewed I/D polymorphism. High-altitude exposes various physiological and biochemical changes, which contributes a rise in systemic blood pressure of the body. There are very few studies available in North-India, with a core focus on the high altitude hypertension. Therefore, a current study supported an interest to find out the association of high altitude hypertension with ACE gene I/D polymorphism.
Methods: to study the significant association with respect to altitude, genetic, biochemical, anthropometric and physio-metric comparison were conducted among 98 individuals where 489nbeing hypertensive patients and other half being normotensive, inclusive of both males and females. The entire results were finally examined and analyzed using statistical software SPSS 16.0 version.
Results: According to study results, mean arterial blood pressure and triglycerides were significantly (p<0.05) associated with SBP among both hypertensive and normotensives. Whereas HDL, LDL-HDL ratio, CHO-HDL were significantly associated only among hypertensive, and age, PP, and SpO2 have been significantly (p<0.05) associated with SBP among normotensive, a strong predictor for SBP.
Conclusion: The genotypic observations were visibly linked with the disease, however, the results were statistically non-significant (ID/DD vs. II; OR: 0.54, 95% CI: 0.20-1.44, p= 0.217). A further study with considerate knowledge of noteworthy dynamics, mainly, altitude, population size, and ethnicity are recommended.
Keywords: altitude, cholesterol, blood pressure, polymorphism, hypertension
ACE, angiotensin converting enzyme; AT1R, angiotensin ii type 1 receptor; AT2R, angiotensin II type 2 receptor; BMI, body mass index; CHO, total cholesterol; CHO-HDL, total cholesterol-high density lipoproteins ratio; CI, confidence interval; D, deletion; DBP, diastolic blood pressure; HDL, high density lipoproteins; HWE, hardy weinberg equilibrium; I: insertion; I/D, insertion/deletion; LDL, low density lipoproteins; LDL-CHO, low density lipoproteins-total cholesterol ratio; MAP, mean arterial blood pressure; PP, pulse pressure; PR, pulse rate; OR, odds ratio; RAS, renin-angiotensin System; SBP, systolic blood pressure; TG: triglycerides; VLDL, very low density lipoproteins; WC, waist circumference; WHR, waist-to-hip ratio
Human essential hypertension accounts for 90% of the hypertensive population, is a complex multifactorial and polygenic disorder1,2 affecting large groups with a genetic heritability ranging from 15% to 35%.3-6 The interplay between environmental and genetic factors is a major determinant of the final phenotype in hypertension.2 Several genes: angiotensinogen gene (AGT), angiotensin I-converting enzyme (ACE), angiotensin II type 1-receptor (AT1-R) and angiotensin II type 2- receptor (AT2-R) have been reported an association with hypertension.7-11 ACE is the major enzyme of the renin-angiotensin-aldosterone system (RAAS), functions conversion of angiotensin I to angiotensin II which binds to plasma membrane receptors, producing arteriolar constriction and a rise in systolic and diastolic blood pressure. ACE is encoded by a 21 kb gene with 26 exons, located on chromosome 17. A polymorphism of ACE gene involves the insertion (I) and deletion (D) of 287 bp Alu repeat sequence near the 3’ end of the intron. Physiologically it has been reported ACE I/D accounts for 50% of the inter-individual variability of plasma ACE concentration.12-14
High-altitude environments imply stress factors: hypoxia, cold, humidity, solar radiation, cosmic radiation and isolation, causing many physiological and biochemical changes in body, including structural changes in the walls of small pulmonary arteries, predominantly increased masculinization, increased pulmonary vascular resistance and sustained elevation of pulmonary arterial pressure, instigating high altitude pulmonary hypertension (HAPH).15-18 In humans, large inter-individual differences exist in the magnitude of the pulmonary pressure response to hypoxia,19-22 with some subjects demonstrating exaggerated increases in pulmonary arterial pressure.23,24 There are very few studies available in North-India where the high altitude hypertension has been focused. Therefore, the objective of this study was designed, supporting an interest to find out the association of high altitude hypertension with ACE gene I/D polymorphism.
Study protocol
This study was a prospective observation of genetic and biochemical analysis among hypertensive patients and normotensive controls, to analyze the significant association of hypertension in concordance to altitude. The study protocol was approved by the institutional board of committee and experiments were performed in the registered department of Human Genetics, Guru Nanak Dev University, and Amritsar, India.
Sample collection
A total of 98 individual samples, 49 hypertensive and 49 normotensives, both males and females included, were collected from high altitude areas of Himachal Pradesh and low altitude areas of Punjab. The patient samples were collected from clinics and hospitals and control samples were taken via door to door study. The patient and control data was collected on a pre-designed Performa, referring to demographic and clinical features. A 3-5 ml of peripheral blood from each individual was withdrawn with a sterile disposable syringe, after having the informed consent. Blood and plasma samples were stored differently for genetic and biochemical analysis. Also, the body Physiometeric measurements of individuals were noted, as useful annotations during statistical analysis of overall data.
Genetic studies
To study ACE I/D Polymorphism, DNA isolation using phenol-chloroform method was performed using the blood samples of individuals from both hypertensive and normotensive groups. After confirming the quality of DNA (min. 50ng/µl) using Agarose gel electrophoresis (ƛ200) and NanoDrop1000 techniques, proceeded the next step of PCR reactions, an enzymatic amplification of DNA fragments (95⁰C- 5 min. and 30 sec denaturation, 56⁰C- 30 sec. annealing, 72⁰C- 30 sec. and 5 min. extension, for 30 cycles), using ACE I/D gene specific primers (F- 5’-CTG GAG ACC ACT CCC ATC CTT TCT -3’, R- 5’-GAT GTG GCC ATC ACA TTC GTC AGA TTT-3’). The amplicons were analyzed for I/D (I at 490bp and D at 190bp) of the 287bp Alu repeats in an ACE gene on 1.5% agarose gel, stained with Ethidium Bromide, using the 100pb DNA marker to study the genotyping pattern of both the groups (Figure 1).
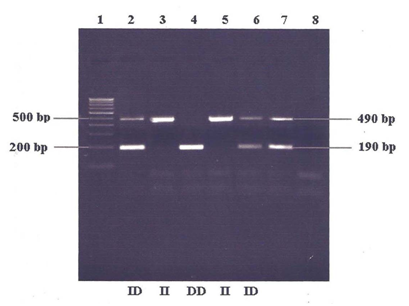
Figure 1 Gel Picture showing I/D polymorphism of ACE gene.
Lane 1: 100 bp DNA ladder
Lane 2-6: Random samples
Lane 7: Positive control
Lane 8: Negative control
Biochemical studies
Total cholesterol (CHO) (desirable <200, borderline 200-239, high >240), triglycerides (desirable <150, borderline 150-199, high 200-500), high density lipopolysaccharides (HDL) (low > 40, high < 50), low-density lipopolysaccharides (LDL) and very low-density lipopolysaccharides (VLDL), HDL-LDL ratios, CHO-LDL ratio were calculated via kit based techniques, using blood plasma separated from the collected blood samples of both groups.
Anthropometric and physiometeric measurements
The height, weight, waist and hip circumference calculated for each individual using standard anthropometric technique and the physio metric variables: systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate, taken 2 hours after meal, using the calculated average of 3-4 time measurements via automatic machine, saturated oxygen pressure (SpO2) using pulse oxy-meter, were calculated for both groups.
Statistics
The data collected from genetic, biochemical, anthropometric and Physiometeric studies were analyzed statistically using SPSS (16.0 version), to evaluate the associated significance in this study. All 98 individuals included, were the only eligible candidates supporting WHO (2009) criteria of normal and hypertensive blood pressure measurements in the conducted study. The analysis included, differential statistical comparisons (for Physiometeric, anthropometric and biochemical variables), linear and multivariate regression (to calculate significant predictors of SBP and DBP), risk estimation (odds ratio, CI-95%, for SBP and DBP), allelic frequency distribution (with respect to ACE genotypes inclusive of risk factors (odds ratio, CI- 95%), Figure 2), for the individuals of both groups. All statistical tests were two-sided with a significance level of 0.05.
In this study, we compared descriptive statistics for different anthropometric, psychometric, biochemical and lifestyle variables with a p-value (p<0.05) of significance among hypertensive and normotensive individuals (Table 1). The study revealed, mean values of age, SBP, DBP, MAP, PP, SpO2, CHO, LDLs and VLDLs were significantly higher (p<0.05) for all the variables, except SpO2, with significant lower p-value among high altitude hypertensive individuals.
Variable |
Hypertensive |
Normotensive |
t |
p-Value |
||||
N |
Mean |
SD |
N |
Mean |
SD |
|||
Age(years) |
49 |
55.02 |
13.52 |
49 |
49.87 |
9.67 |
2.088 |
<0.042 |
Height(cm) |
49 |
1.62 |
0.085 |
49 |
1.64 |
0.09 |
0.894 |
0.376 |
Weight(kg) |
49 |
71.09 |
12.26 |
49 |
69.56 |
10.52 |
0.730 |
0.469 |
Body mass index(BMI)(kg/m2) |
49 |
26.96 |
4.27 |
49 |
25.93 |
3.54 |
1.375 |
0.176 |
Waist circumference(cm) |
49 |
96.44 |
9.96 |
49 |
93.26 |
10.75 |
1.568 |
0.124 |
Hip circumference(cm) |
49 |
99.43 |
8.38 |
49 |
99.16 |
7.97 |
0.166 |
0.869 |
Waist-hip ratio |
49 |
0.97 |
0.06 |
49 |
0.94 |
0.08 |
1.861 |
0.69 |
Systolic blood pressure(mmHg) |
49 |
146.55 |
17.64 |
49 |
130.12 |
13.22 |
5.097 |
<0.000 |
Diastolic blood pressure(mmHg) |
49 |
90.32 |
10.06 |
49 |
79.97 |
9.09 |
5.327 |
<0.000 |
MAP(mmHg) |
49 |
109.07 |
11.57 |
49 |
96.69 |
9.80 |
5.557 |
<0.000 |
Pulse Pressure(mmHg) |
49 |
56.22 |
12.96 |
49 |
50.14 |
8.84 |
2.839 |
<0.007 |
Pulse rate(counts/min) |
49 |
77.65 |
14.47 |
49 |
78.16 |
9.83 |
0.185 |
0.854 |
SpO2 (%) |
49 |
97.20 |
1.92 |
49 |
97.91 |
0.73 |
2.449 |
<0.018 |
Alcohol |
49 |
1.61 |
0.88 |
49 |
1.43 |
0.67 |
1.176 |
0.245 |
Smoking |
49 |
1.48 |
0.86 |
48 |
1.25 |
0.56 |
1.631 |
0.110 |
Exercise |
49 |
0.73 |
0.49 |
48 |
0.58 |
0.57 |
1.533 |
0.132 |
Food habit |
49 |
1.48 |
0.50 |
49 |
1.38 |
0.49 |
1.000 |
0.322 |
Physical fitness |
49 |
1.89 |
0.30 |
49 |
1.93 |
0.31 |
0.629 |
0.533 |
Total-cholesterol (CHO)(mg/dl) |
41 |
207.02 |
88.40 |
49 |
153.46 |
50.39 |
2.933 |
<0.006 |
Triglycerides(mg/dl) |
41 |
199.26 |
129.38 |
49 |
193.59 |
103.54 |
0.017 |
0.987 |
High density lipoproteins(mg/dl) |
41 |
50.56 |
28.81 |
49 |
45.22 |
32.45 |
0.742 |
0.463 |
Low density lipoprotein(mg/dl) |
41 |
116.59 |
94.89 |
49 |
69.52 |
48.64 |
2.528 |
<0.016 |
Very low density lipoprotein |
41 |
39.85 |
25.87 |
49 |
38.71 |
20.70 |
3.464 |
<0.001 |
LDL-HDL ratio |
41 |
3.00 |
2.69 |
49 |
2.03 |
1.69 |
1.616 |
0.114 |
CHO-LDL ratio |
41 |
5.01 |
2.72 |
49 |
4.15 |
2.25 |
1.192 |
0.240 |
Table 1 Descriptive statistics for different studied variables among Hypertensive and Normotensive groups
HDL, high density lipoprotein, LDL, low density lipoproteins
Regression models
The descriptive results were further continued by calculating significant predictors of SBP and DBP through univariate regression analysis. We found during analysis that DBP, MAP, and PP are significantly associated (p<0.05) with SBP among both groups whereas height and weight are significantly associated (p<0.05) with SBP among normotensive individuals. It was also found during analysis that MAP and triglycerides were significantly associated (p<0.05) with SBP among hypertensive and normotensive individuals. HDL, LDL-HDL ratio, and CHO-HDL ratios were significantly associated among hypertensive only and on the other hand age, pulse pressure, and SpO2 were significantly associated (p<0.05) with SBP among low altitude normotensive and therefore strong predictor for SBP (Table 2). Table 3 describes, LDL-HDL ratio and CHO-HDL are significantly associated (p<0.05) among hypertensive individuals whereas age, mean arterial pressure and pulse pressure were found significantly associated (p<0.05) among normotensive individuals with DBP.
Variables |
High Altitude Hypertensive |
Low Altitude Normotensive |
||||||
Coefficient |
Std. Error |
t |
p-Value |
Coefficient |
Std. Error |
t |
p-Value |
|
Age(years) |
0.370 |
0.224 |
1.655 |
0.105 |
0.400 |
0.189 |
2.12 |
<0.040 |
Height(cm) |
-1123.1 |
806.08 |
1.393 |
0.170 |
160.92 |
105.83 |
1.52 |
0.136 |
Weight(kg) |
0.406 |
0.366 |
1.107 |
0.274 |
-1.13 |
1.275 |
0.893 |
0.377 |
Body mass index(BMI)(kg/m2) |
-0.384 |
1.258 |
0.305 |
0.762 |
4.498 |
3.401 |
1.323 |
0.193 |
Waist circumference(WC)(cm) |
0.729 |
0.528 |
1.380 |
0.174 |
-0.364 |
0.257 |
1.418 |
0.163 |
Hip circumference(cm) |
0.001 |
0.001 |
1.288 |
0.204 |
-0.001 |
0.005 |
0.215 |
1.053 |
Waist-hip ratio |
0.008 |
0.0198 |
0.437 |
0.664 |
0.045 |
0.005 |
0.903 |
0.371 |
Mean arterial pressure(mmHg) |
3.00 |
0.02 |
10.3 |
<0.001 |
1.000 |
0.004 |
22.43 |
<0.001 |
Pulse pressure(mmHg) |
1.149 |
0.117 |
9.804 |
1.043 |
0.667 |
0.004 |
13.24 |
<0.001 |
Pulse rate(counts/min) |
0.117 |
0.011 |
1.033 |
0.307 |
0.134 |
0.188 |
0.711 |
0.481 |
SaO2 (%) |
0.602 |
0.859 |
0.700 |
0.487 |
-5.76 |
2.486 |
2.321 |
<0.025 |
Total cholesterol(CHO)(mg/dl) |
0.002 |
0.030 |
0.095 |
0.924 |
-0.014 |
0.042 |
0.340 |
0.735 |
Triglycerides(mg/dl) |
0.040 |
0.020 |
1.984 |
<0.050 |
0.033 |
0.019 |
1.848 |
<0.05 |
HDL(mg/dl) |
-0.182 |
0.092 |
1.958 |
<0.050 |
-0.095 |
0.063 |
0.015 |
0.988 |
LDL(mg/dl) |
0.0405 |
0.053 |
0.763 |
0.450 |
-0.034 |
0.060 |
0.578 |
0.566 |
VLDL(mg/dl) |
-0.09 |
0.157 |
0.595 |
0.556 |
0.191 |
0.141 |
1.350 |
0.184 |
LDL-HDL ratio |
-14.08 |
6.127 |
2.299 |
<0.027 |
1.864 |
4.428 |
0.421 |
0.676 |
CHO-HDL ratio |
12.9 |
4.978 |
2.593 |
<0.014 |
-0.827 |
2.942 |
0.281 |
0.780 |
Table 2 Calculation of significant predictor of systolic blood pressure (SBP) through multivariate regression analysis among high altitude hypertensive and low altitude low altitude normotensive individuals
HDL, high-density lipoproteins; LDL, low-density lipoproteins; VLDL, very low-density lipoproteins; R2: percent of variance
|
High Altitude Hypertensive |
Low Altitude Normotensive |
||||||
Variables |
Coefficient |
Std. Error |
T |
p-Value |
Coefficient |
Std. Error |
t |
p-Value |
Age(years) |
0.093 |
0.144 |
0.647 |
0.522 |
0.009 |
0.004 |
2.094 |
<0.042 |
Height(cm) |
39.60 |
20.95 |
0.078 |
0.938 |
27.18 |
14.16 |
1.919 |
0.062 |
Weight(kg) |
0.017 |
0.222 |
0.807 |
0.424 |
0.349 |
0.188 |
1.853 |
0.071 |
Body mass index(BMI)(kg/m2) |
0.059 |
0.812 |
0.073 |
0.942 |
-0.80 |
0.576 |
1.399 |
0.169 |
Waist circumference(WC)(cm) |
3.43 |
2.218 |
1.547 |
0.129 |
-0.28 |
1.918 |
0.148 |
0.883 |
Hip circumference(cm) |
-3.37 |
2.009 |
1.679 |
0.100 |
0.459 |
1.730 |
0.265 |
0.792 |
Waist-hip ratio |
-31.4 |
20.60 |
1.413 |
0.165 |
14.52 |
18.742 |
0.076 |
0.939 |
Mean arterial pressure(mmHg) |
31.6 |
25.01 |
1024 |
0.625 |
1.00 |
0.0043 |
23.89 |
<0.001 |
Pulse pressure(mmHg) |
0.149 |
0.117 |
1.274 |
0.209 |
-0.33 |
0.0051 |
6.139 |
<0.001 |
Pulse rate(counts/min) |
0.117 |
0.113 |
1.033 |
0.307 |
0.002 |
0.0040 |
0.707 |
0.483 |
SaO2 (%) |
0.602 |
0.859 |
0.700 |
0.487 |
0.002 |
0.0057 |
0.452 |
0.654 |
Total cholesterol(CHO)(mg/dl) |
0.05 |
0.019 |
0.306 |
0.761 |
-0.04 |
0.0280 |
1.580 |
0.121 |
Triglycerides(mg/dl) |
0.22 |
0.012 |
1.758 |
0.087 |
0.021 |
0.0127 |
1.717 |
0.093 |
HDL(mg/dl) |
-0.05 |
0.058 |
0.897 |
0.375 |
-0.042 |
0.0421 |
1.018 |
0.314 |
LDL(mg/dl) |
0.07 |
0.018 |
0.391 |
0.698 |
-0.025 |
0.0413 |
0.618 |
0.540 |
VLDL(mg/dl) |
0.115 |
0.069 |
1.668 |
0.104 |
-0.024 |
0.0982 |
0.250 |
0.804 |
LDL-HDL ratio |
-4.00 |
2.041 |
1.959 |
<0.05 |
-0.913 |
2.987 |
0.030 |
0.761 |
CHO-HDL ratio |
4.422 |
2.013 |
2.197 |
<0.035 |
1.343 |
1.985 |
0.677 |
0.502 |
Table 3 Calculation of significant predictor of diastolic blood pressure (DBP) through multivariate regression analysis among hypertensive and normotensive individuals
HDL, high-density lipoproteins; LDL, low-density lipoproteins; VLDL, very low-density lipoproteins; R2, percent of variance
ACEallele and genotype with different genetic inheritance models between high altitude hypertensive cases and low altitude normotensive controls was shown in Table 4. The genotype and allele distributions of the ACE gene are almost similar and did not differ significantly between control and cases. No deviation from HWE has been found within both case and control group separately. However, there was a suggestive evidence of an association in a recessive model (OR: 0.83, 95% CI: 0.33-2.05, p= 0.681). It was also evident from the study that dominant model of inheritance has some protective impact with respect to high altitude hypertension but, the result found were not statistically significant (OR: 0.54, 95% CI: 0.20-1.44, p= 0.217) (Table s1 $ s2).
Total N (control + case)=88 |
|||||||||||||||
Study |
Genotype (%) |
Allele (%) |
p-Value |
Dominant Model |
Co-Dominant Model |
Recessive Model |
Test for H.W. |
||||||||
II |
ID |
DD |
I |
D |
Genotype |
Allele |
OR |
p-Value |
OR |
p-Value |
OR |
p-value |
x2 |
P |
|
Control |
15 |
22 |
9 |
54 |
40 |
0.46 |
0.306 |
0.54 |
0.217 |
0.76 |
0.331 |
0.83 |
0.681 |
0.033 |
0.855 |
Case |
12 |
17 |
13 |
41 |
43 |
1.516 |
0.218 |
||||||||
Table 4 Distribution of frequencies of Angiotensin Converting Enzyme (ACE) genotypes, alleles and genetic models in High Altitude Hypertensive Cases and Low Altitude normotensive Controls. Data are a number of subjects with each genotype and allele (frequency in percentage). OR- Odds Ratio, CI- Confidence Interval. ORs for different modes of inheritance have been calculated
The study described the association between the ACE variant of high altitude hypertension in North Indian population. Many studies have shown a significant ACE gene D allele with essential hypertension.25-31 On the other hand, several researchers have shown no significant differences in the allele and genotype distribution of ACE gene polymorphism between low altitude normotensive control and high altitude hypertensive cases.32-39 The increase in body size has seen to be positively associated with blood pressure. There is a hypothesis that high body weight individuals are more likely to develop systemic hypertension at high altitude. On exposure to high altitude, systemic hypertension results from sympathetic stimulation and it may continue for many weeks. The involvement of the renin-angiotensin-aldosterone system (RAAS) in the control of the salt and water balance and thereby blood pressure is well known during hypoxia and high altitude exposure.35,36 At high altitude renal secretion is stimulated by decreased renal blood flow,37 which in turn activates the RAAS.16
In the present study, significant differences were observed in hypertension parameters such as pulse pressure, total cholesterol, low-density lipoproteins and very low-density lipoproteins. The mean values of all these parameters have been observed higher in high altitude hypertensive individuals, however, the content of O2 saturation (SpO2) has been higher in low-altitude individuals. In the present study of a small data set, the overall frequencies of the risk allele (D) have been higher in high altitude hypertensive individuals but, not significant (p =0.47) as compared to low altitude normotensive individuals. Therefore, D allele association hypothesis of hypertension in high altitude have not reflected to be true in the present study. The same also is true for I allele. The different covariates of hypertension such as body mass index, waist-hip ratio, waist circumference, hip circumference etc. have also not seen significantly different between high altitude hypertensive individuals. This observation also strengthens the present genotypic association analysis which did not show any association between ACE gene polymorphism in hypertension in high altitude cases.
In some population, the I allele may be in linkage disequilibrium with a mutation elsewhere in the gene, whereas in other population the D allele might be in linkage disequilibrium with the different ACE mutation. This might explain the association of essential hypertension and ACE-I allele. The recently described variety of potentially functionally variants in the ACE gene may support the alternative hypothesis.38-40 However, studies in North Indian Punjabi41-45 reported that diseases such as central obesity, type-2 Diabetes Miletus, and hyperlipidemia are more common but they have a low risk of developing cardiovascular diseases due to their strong genetic background. The low frequency of ACE DD genotype in this population might provide a protective effect for cardiovascular diseases. However, in this regard, not many data are available to support the present findings of this population.46
The present analysis suggests that this ACE gene polymorphism has no major influence on the susceptibility to elevate blood pressure phenotype with respect to high altitude. In the meantime, it should be noted that the present study has been carried out on a small sample size; despite this fact the findings which have been in homogenous population base study cannot be ignored.
The study was carried as the thesis work of a Master’s student, therefore limited the number of subjects to be enrolled due to the short duration of time. Although the places were chosen were new for the study but the altitude was not really that higher to prove a significant association even in the lesser number of samples. It might have been more convincing with a better knowledge of population statistics and a larger group of cases enrolled. Though the results in conducted study could not give expected statistics but the non-statistical genotypic frequency results observed has given a supportive overview of ACE gene association with High altitude hypertension. If the study is conducted further with a larger data groups the results might be as promising as expected.
We gratefully acknowledge the study investigators, fellow researchers, clinicians, and patients and individuals who volunteered to participate in the study. The supported funding and work were done in the department of Human Genetics, Guru Nanak Dev University, and Amritsar, India. The work was re-analyzed by S.C and edited by K.D at institute of renal sciences and research, Sir Ganga Ram Hospital. Delhi, India.
None.

©2017 Chandel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.