eISSN: 2378-3176


Research Article Volume 12 Issue 3
Department of Nephrology, Hospital Ernesto Dornelles, Brazil
Correspondence: Cinthia Kruger Sobral Vieira, Department of Nephrology, Hospital Ernesto Dornelles, Brazil, Tel +5551992491700
Received: October 03, 2024 | Published: October 16, 2024
Citation: Logemann P, Vieira GS, Pereira JD, et al. Analysis of the renal biopsy database of patients from a nephrology and dialysis center. Urol Nephrol Open Access J. 2024;12(3):61-64. DOI: 10.15406/unoaj.2024.12.00358
Renal biopsy is an essential tool for diagnosing many conditions that affect the kidneys. It is the gold standard test for detecting glomerulopathies and parenchymal renal disease and, therefore, helps determine the best treatment for the diagnosed disease.1–4
Percutaneous renal biopsy, commonly performed under ultrasound guidance, is the most commonly used approach, as it is less invasive and can be performed on an outpatient basis.
Because of the invasive nature of the procedure, complications are not uncommon.
This study aims to analyze the results of biopsies collected at a tertiary referral center in Brazil. We evaluated whether the quality of the tissue sample obtained was sufficient to diagnose the focal lesion and, therefore, establish a diagnosis and treatment for the patient. An epidemiological analysis of the patients undergoing the procedure was also performed, as well as a survey of the main complications related to it.
Keywords: renal biopsy, kidney, epidemiology, diagnosis
The kidney biopsy is an invaluable tool that has become the gold standard for the diagnosis of pathologic kidney diseases since the early 1950s.
A kidney biopsy has become a preferred method to obtain critical information that can be used in conjunction with serologic, urinary, and genetic testing to diagnose a variety of kidney diseases, both acute and chronic.1
Immunohistologic and ultrastructural microscopy techniques have improved and provide more information on the cause and classification of kidney disorder and, with this, helps to determine the best treatment for the diagnosed disease.
Percutaneous renal biopsy is the most commonly used approach, since it is less invasive and can be performed on an outpatient basis. The technique of this procedure has evolved significantly in recent years and is now commonly performed under real-time ultrasound guidance and, in more complex cases, under computed tomography guidance. In these circumstances, since image quality has improved substantially, it is possible not only to increase the diagnostic yield through adequate tissue samples but also to reduce the complications of this procedure.1,5
Potential complications include bleeding requiring transfusion, gross hematuria, arteriovenous fistula formation, and perinephric hematoma, among others.1
For a high diagnostic yield, it is essential to obtain an adequate biopsy specimen. Satisfactory tissue is usually obtained in more than 90% of biopsy attempts.4,6
There is currently no absolute number of glomeruli required in a sample to make a diagnosis, but the greater the number of glomeruli, the lower the risk of missing a focal lesion. For example, a biopsy from a patient with nephrotic syndrome secondary to FSGS has a 35% chance of missing a segmental scar on light microscopy if only 10 glomeruli are sampled and segmental scars are present in 10% of the glomeruli. However, if 20 glomeruli are sampled, the statistical probability of missing a segmental lesion drops to 12% (Corwin et al. 1988). Based on these statistical analyses, it is ideal to collect a sample that contains at least 10 or more glomeruli for evaluation. Biopsies with fewer glomeruli can and should still be interpreted, but the possibility of sampling error should be considered.7
In patients with contraindications to the percutaneous approach, such as failure to obtain adequate radiographic visualization or bleeding diathesis, alternative methods of tissue acquisition have been used. These include open, laparoscopic, transurethral, or transvenous (transjugular and transfemoral) renal biopsy.1,8
When there is a contraindication to percutaneous renal biopsy, many factors must be considered before deciding on the best method to perform the procedure. Safety, morbidity, recovery time, likelihood of sample adequacy, and the experience of the physician performing the technique are variables that must be evaluated when a tissue diagnosis is required to impact treatment.1
This retrospective and observational study included all patients submitted to percutaneous native kidney biopsy at the Centro de Nefrologia e Diálise do Hospital Ernesto Dornelles, a tertiary nephrology center in southern Brazil from January 1st 2022 to July 31st 2024.
The inclusion criteria for the study were patients with native kidneys who underwent biopsy using the percutaneous method and who presented some degree of hematuria, proteinuria or unexplained loss of renal function.
All kidney biopsies were performed by nephrologists with ultrasound guidance. There were no significant changes in biopsy method during this time period.
Patients with transplanted kidneys and/or renal tumor masses were excluded from the study, as well as those who underwent renal biopsy using some other methodology, such as trans jugular, laparoscopic, or surgical.
Samples were examined by experienced pathologists and were considered valid if there were at least 1 glomeruli or otherwise if a diagnosis was made, according to institutional protocol and literature.
Immunofluorescence staining using polyclonal antisera against human IgG, IgM, IgA, C3c, C4c, C1q, Fibrinogen, Kappa and Lambda was performed.
Renal diseases were divided into four groups: 1) primary glomerulonephritis (GN); 2) secondary GN; 3) tubulointerstitial diseases, and 4) other diseases. Primary GN included: crescent glomerulonephritis (CreGN), focal segmental glomerulosclerosis (FSGS), idiopathic membranous nephritis, IgAN, membranoproliferative glomerulonephritis (MPGN), mesangial proliferative glomerulonephritis (MsPGN) and Minimal Change Disease (MCD).
Secondary GN included acute post-infectious glomerulonephritis (APiGN), IgAVN, lupus nephritis (LN) and renal amyloidosis. Tubulointerstitial diseases included acute and chronic tubulointerstitial nephritis. Other diagnoses comprise thrombotic micro-angiopathy (TM).
Medical records of patients who underwent renal biopsy were reviewed using the Phillips® Tasy electronic system at Hospital Ernesto Dornelles. Data such as the patients' epidemiological profile, associated comorbidities, renal function on the date of the procedure, and occurrence of complications after the procedure were collected.
Data were entered into Excel and later exported to SPSS v. 20.0 for statistical analysis. Categorical variables were described by frequencies and percentages and quantitative variables by mean and standard deviation.
This study was approved by the institution’s Ethics Committee and individual informed written consent was deemed unnecessary for this retrospective and observational study.
Thirty patients who underwent kidney biopsy (KB) at the Centro de Nefrologia e Diálise do Hospital Ernesto Dornelles from January 2022 to July 2024 were analyzed.
Demographic characteristics are represented in Table 1. In our sample, 46,7% were male (n = 14) and 53,3% of patients were female (n = 16). Median age at time of biopsy was 56 (15 - 84).
|
Characteristic participants |
(N = 30) |
|
Sex - no. (%) |
|
|
Male |
14 (46,7) |
|
Female |
16 (53,3) |
|
Age - yr |
|
|
Mean |
56,86 +/- 17,07 |
|
Median (range) |
61,05 (15 - 84) |
|
Comorbidities - no. (%) |
|
|
Hypertension |
17 (56,7) |
|
Chronic kidney disease |
11 (36,7) |
|
Diabetes mellitus |
8 (26,7) |
Table 1 Demographic and clinical characteristics of the participants
Indications for percutaneous KB and distribution are represented in Figure 1.
The most common indication for KB in the overall sample was nephrotic proteinuria in 53,3% of patients (n = 16), followed by acute kidney injury in 20% (n = 6) and asymptomatic urinary abnormalities in 13,3% (n = 4).
A median of 19 (7 – 40) glomeruli were obtained. Of the 30 anatomopathological results analyzed, we observed that 50% of patients (n = 15) had more than 20 glomeruli in their examination, 40% of patients (n = 12) had a total of 10 to 20 glomeruli in the tissue sample, and only 10% of patients (n = 3) had less than 10 glomeruli in their examination.
Immunofluorescence was performed in 96,6% of cases. Electron microscopy was not performed since in all cases the findings in optical microscopy were conclusive. In addition to this reason, access to electron microscopy is more restricted in our region since the analyses must be performed in other states.
We found that 100% of the patients biopsied at the unit had a renal biopsy that was conclusive, that is, that provided some diagnosis to the patient. In addition, one of the thirty patients presented a complication related to the procedure.
IgA nephropathy (IgAN) was the most common pathology found (23,3%, n=7), followed by renal amyloidosis (13,3%, n =4), primary membranous glomerulonephritis (10%, n=3), pauci-immune crescentic glomerulonephritis (10%, n=3) and diabetes kidney disease (10%, n=3). Secondary membranous glomerulonephritis and hypertensive nephrosclerosis accounted for 6,7% each; minimal change disease (MCD), monoclonal gammopathy of renal significance (MGRS), thrombotic microangiopathy, lupus glomerulonephritis, secondary membranoproliferative glomerulonephritis and acute interstitial nephritis accounted for 3,3% each.
Figure 2 shows the relative distribution of the most common pathologies.
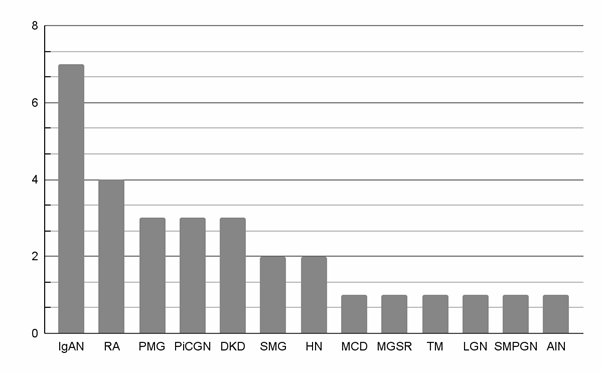
Figure 2 Distribution of pathologies
IgAN, IgA nephropathy; RA, Renal amyloidosis; PMG, Primary membranous glomerulonephritis; PiCGN, Pauci-immune crescentic glomerulonephritis; DKD, Diabetes kidney disease; SMG, Secondary membranous glomerulonephritis; HN, Hypertensive nephrosclerosis; MCD, Minimal change disease; MGRS, Monoclonal gammopathy of renal significance; TM, Thrombotic microangiopathy; LGN, Lupus glomerulonephritis; SMPGN, Secondary membranoproliferative glomerulonephritis; AIN, Acute interstitial nephritis.
Among patients with nephrotic proteinuria, renal amyloidosis and primary membranous glomerulonephritis were more frequently observed (25%, each).
Among patients up to 45 years old, the most common pathology was IgAN (n=5, 55,5%). In patients between 45 and 64 years old, the most common pathologies were renal amyloidosis (n = 3, 33,3%) and primary membranous glomerulonephritis (n=3, 33,3%).
In elderly (65 years old or older), the most common pathology was pauci-immune crescentic glomerulonephritis (n = 3, 25%), hypertensive nephrosclerosis (n = 2, 16,66%) and diabetes kidney disease (n=2, 16,66%).
This study presents our department’s experience in percutaneous kidney biopsy performed by nephrologists with ultrasound guidance over the last 2 years.
Based on statistical analyses, it is ideal for the renal biopsy procedure to contain a sample with at least 10 or more glomeruli to perform the evaluation. Biopsies with a smaller number of glomeruli can and should still be interpreted, but the possibility of sampling error should be taken into account.7 This study showed that the vast majority of biopsies performed in the unit presented an adequate tissue sample and with a number of glomeruli capable of generating a definitive diagnosis, suggesting satisfactory technical quality. A total of 90% of the anatomopathological findings presented 10 or more glomeruli in the anatomo pathological findings.
Primary glomerular diseases were more frequent than secondary GN, as reported in several studies.
According to Magistroni et al., IgA nephropathy (IgAN) is recognized as the most frequent form of idiopathic glomerulonephritis worldwide.7 Likewise, in this study we observed that this pathology was also the most prevalent, with a total of 23,3% of all diagnoses found.
A significant number of patients diagnosed with renal amyloidosis were also observed (n=4, 13,3%), mainly in patients under 65 years of age, as opposed to studies which found a prevalence of approximately 2% of renal biopsies.9
The four patients were diagnosed with AL renal amyloidosis and none of these patients met the criteria for a definitive diagnosis of multiple myeloma. Although AL amyloidosis is the result of clonal proliferation of plasma cells, most patients do not meet criteria for multiple myeloma, likewise in this study. These patients are best categorized as having monoclonal gammopathy of renal significance.10
Primary Membranous Glomerulonephritis (PMG) is the main cause of nephrotic syndrome in non-diabetic white adults (about 30%), with an estimated annual incidence of 10 – 12 cases per million/year in the North American population.11 In Brazil, considering primary glomerulopathies, PMG is the second most frequent diagnosis in native kidney biopsies (20.9%).12 Interestingly in our study, 10% of cases corresponded to patients diagnosed with primary membranous glomerulonephritis, all cases with antibodies phospholipase A2 receptor (PLA2R) associated.
The study carried out by Guerrero-Ramos et al. reported a complication rate after percutaneous renal biopsies of 5.6%, with interventional intervention being necessary in only 1.67% of cases. These findings are similar to those of Ali et al., who evaluated 527 ultrasound-guided renal biopsies and reported an overall complication rate of 5.64% and a major complication rate of 2.84%.12,13
In this study, one of the thirty patients (n = 1, 3,33%) presented a complication related to the procedure.
In this case, the formation of a perirenal hematoma was identified by ultrasound immediately after the puncture, and the patient was promptly referred to the emergency department and serial CT scans were performed to assess active bleeding. No surgical intervention or transfusion of blood products was necessary and the patient was discharged from hospital 4 days after the renal biopsy.14
This low number of complications may indicate that pre- and post-procedure measures were performed appropriately to prevent complications, but this result may be masked by the small sample size.
The main limitation of this study was its small sample size in a single center, which restricted the possibility of comparative analyses with the literature and the performance of statistical associations; also the single-center cohort design may potentially limit the external validity of our results.
Nevertheless, this study confirms the reliability of percutaneous KB as a diagnostic tool which can probably impact the management and hence improve the outcome.
All authors contributed to the study conception and design. Data collection was performed by PL and CJ. Sample analysis was performed by PL and CJ. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
None.
Nothing to declare, including financial conflicts of interest.

©2024 Logemann, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.