eISSN: 2378-3176


Conn’s adenoma on a field of celiac disease has never been described in the literature. We report a case of 31-year-old woman with a history of celiac disease under gluten-free diet, and a pregnancy-induced hypertension controlled under treatment. She was presented with hypertension crisis, hypokalemia and metabolic alkalosis. Diagnosis of primary aldosteronism was suspected, which was confirmed by a decrease plasma renin, elevated plasma aldosterone level and high aldosterone/renine ratio additionally. Adrenal computed tomography revealed left adrenal mass. The patient was treated with nicardipine, spironolactone and potassium supplements. Laparoscopic left adrenalectomy was performed. The final diagnosis was adenoma of the adrenal gland consistent with Conn’s adenoma. Blood pressure and hypokalemia returned to the normal level without any medication or supplement.
Keywords: conn’s adenoma, primary aldosteronism, hypertension, hypokalemia metabolic alkalosis
The aldosterone-producing adenoma (APA), the second most important cause of primary aldosteronism (PA), was first reported by Conn in 1955.1,2 Currently, it is one of the few potentially curable causes of secondary arterial hypertension. It’s a clinical syndrome characterized by hypertension, hypokalemia by excessive urinary excretion of potassium, and metabolic alkalosis. We report a case of a woman with a history of celiac disease with evolving pregnancy-induced hypertension one year ago, who had satisfactorily controlled over blood pressure and developed secondary hypertension with a hypertension crisis, secondary to an APA.
E.O. was a 31-year-old female, with a history of celiac disease under gluten-free diet, and a pregnancy-induced hypertension one year ago, without having been screened for secondary hypertension, to the best of our knowledge. A satisfactory blood pressure (BP) has been reached under treatment with nicardipine (100mg/day). The patient was admitted to our hospital because of headache, muscle weakness and heart palpitations, for two weeks. On physical examination, the patient was in regular general condition and with a healthy coloring. Her weight was 53.5 kg and height 162 cm (BMI=20.41Kg/m2). Blood pressure measured with a manual armlet 190/110 mmHg, heart rate 100beats/minute and regular, respiratory rate 18cycles/min, body temperature 36.9°C. On auscultation, her lungs were clear with no rales. Her heart auscultation showed regular cardiac rhythm with cardiac sounds of normal intensity and no murmurs. Her lower limbs showed symmetric palpable pulses and no edema. The power and sensation of both lower and upper limbs were normal. The abdominal examination showed hydro-aerial noise, no visceromegaly and no abdominal murmur. Thyroid gland examination was normal. There was neither obesity nor hirsutism. Other physical examination was normal. Laboratory findings were as follows: serum potassium 1.68mEq/L (normal range (NR)= 3.5-5.1), sodium 143 mEq/L (NR=136-145), alkaline reserve 32mEq/L(NR=22-31), corrected calcium 95mg/L(NR=86-100), phosphorus 29.9mg/L (NR=25-45), urea 0.15g/L (0.13-0.45), creatinine 3.9mg/L(NR=5-9). The complete blood count and coagulation indices were normal. The thyroid function test was normal (0.94mU/L; NR=0.27-4.2). The measurements of sodium and potassium in the 24-hour urine were 54mEq/L (NR=54-190) and 63 mq/L (NR=25-125), respectively. The ECG showed presence of changes of hypokalemia, U waves and no hypertension changes. Echocardiography assessment of hypertension was negative, and there was no hypertension retinopathy. She was treated with boluses of intravenous potassium chloride and oral potassium supplements, for 260 mmol/day over 6 days. Initially her hypertension was treated with intravenous nicardipine utilizing an electrical syringe pump. Despite intravenous and oral potassium supplementation, her serum potassium level remained low. The PA was suspected because of persistence hypokalemia in association with hypertension and alkalosis. However, after correction of serum potassium level, the patient was examined for the plasma aldosterone concentration (PAC) and direct rennin concentration (DRC). Results revealed a low DRC level of 0.9mUI/L (NR=2.8-39.9), along with a very high PAC level of 2613pmol/l (NR=83-405). The aldosterone to rennin ratio (ARR) was 523pmol/l per mUI/l (N<64). Subsequently, after a three-day oral sodium load, 24-hour urine was tested for the concentration of sodium (260mmols) and aldosterone (27.3mg). Abdominal computerized tomography (CT) revealed a 28x21 mm hypo-dense mass (1UH of density), not enhanced by the contrast product, over the left adrenal gland (Figures 1) (Figures 2). After using spironolactone 100 mg/day and nicardipine 150 mg/day for one month preoperatively, the BP normalized (BP=125/85 mmHg), the headache, weakness and heart palpitations significantly improved, and the serum level of potassium was 4.5 mEq/L. Therefore, the patient underwent a successful laparoscopic left adrenalectomy (Figure 3), and the postoperative course was well. The macroscopic tumor consisted of an ovoid piece of yellow color tissue with weight: 28gr, circumscribed mass: 20x30mm in dimensions adjacent to adrenal tissue. Histologically, the adrenal mass showed an encapsulated epithelial neoplasia with cells of clear cytoplasm, round nuclei and conspicuous nucleoli. However, necrosis, capsular invasion and atypical mitosis were not found (Figure 4). The anatomic pathological diagnosis was adenoma of the adrenal gland consistent with Conn’s adenoma. The patient was normotensive and serum potassium was 4.5mEq/L without any antihypertensive medication or supplement for the last three months after discharge. She was stable with a optimal control of blood pressure without hypokalemia with a 2-year follow-up.
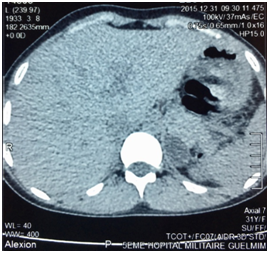

A B
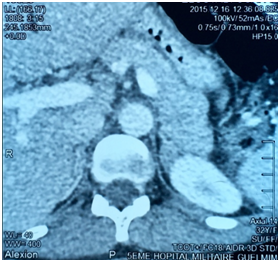
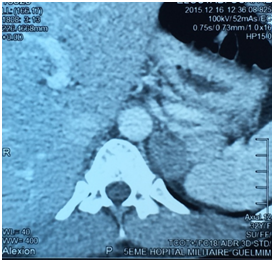
A B
PA is the cause of approximately 0.05-2% of all secondary arterial hypertension is characterized by suppressed plasma renin activity and hypokalemia.3 Conn estimated the prevalence of the disorder around 10% of the general population.4 PA includes unilateral adenoma (=APA) (30%), unilateral (2%) and bilateral (60%) adrenal hyperplasia, familial aldosteronism (1%) and adrenal carcinoma with aldosterone hyper secretion (<1%). The uncontrollable synthesis of aldosterone leads to increased sodium reabsorption, kaliuresis and renin suppression. All of the above challenge arterial hypertension, which affects target organs (heart, kidneys, brain) more gravely than essential hypertension does.5 The APA is a small nodule (< cm) that mostly occurs in the left adrenal gland commonly found in females, and usually present with severe hypertension and more profound hypokalemia. It is also more common in younger patients (30-50 years of age), with higher plasma and urinary levels of aldosterone.6 Some patients are completely asymptomatic or have minimum symptoms resulting from hypertension (headache) and hypokalemia (polyuria, nocturia, muscle cramps). Occasionally, excessive muscle weakness, paresthesias, tetany, and even muscle paralysis may occur.2,7,8 Usually, PA is characterized by hypertension, hypokalemia, excessive urinary excretion of potassium, hypernatremia, and metabolic alkalosis.7 It is recommended testing for the PA in the following groups: patients with hypertension and hypokalemia, treatment-resistant hypertension (three antihypertensive drugs and poor control), severe hypertension (>160 mmHg systolic or>100mmHg diastolic), hypertension and an incidental adrenal mass, and onset of hypertension at a young age.6 We described a case of young (31 year-old) female with a pregnancy-induced hypertension one year ago, without having screened for secondary hypertension, and with satisfactory control of blood pressure under treatment with nicardipine, which favors the initial diagnosis of controlled primary hypertension, despite its beginning at an age suggesting secondary hypertension. The occurrence of hypertension crisis with headaches, muscle weakness, heart palpitations, and significant hypokalemia led us to consider a cause of hypertension secondary in origin superimposed on primary hypertension. Despite intravenous and oral potassium supplementation, her serum potassium level remained low. The PA was suspected because of persistence hypokalemia in association with severe hypertension and alkalosis. Hypokalemia (serum potassium ≤3.5mmol/l) is only present in a minority of patients with PA.9 However; the frequency of hypokalemia is related to whether PA can be surgically cured. A study with a large series of patients reported that hypokalemia was present in 7%, 17% and 48% of patients with essential hypertension, idiopathic PA, and APA, respectively.10 Our patient had an association of PA secondary to APA, and celiac disease under gluten-free diet. This association has never been described in the literature. Both diseases can manifest as hypokalemia, PA by excessive urinary excretion of potassium, and celiac disease by mal absorption, which could trigger or aggravate hypokalemia. The diagnostic approach of PA can be consisted in three steps: the ARR calculation, confirmatory tests of PA and distinction of the disease subtypes. Recent guidelines suggest screening for PA patients with difficult-to-treat or hypokalemic hypertension using the ARR.9 The ARR was introduced by K Hiramatsu and colleagues in 1981 as a screening tool to facilitate the diagnosis of PA among hypertensive patients.11 Although, using the ARR decreases the intra- and inter-patient variability in renin and aldosterone levels linked to sodium intake, body position and age.12,13 The most frequently used cut-off values for PAC to PRA ratios are in the range of 20 to 50ng/dl (554 to 1.385pmol/l) per ng/ml.h; for PAC to DRA, these values are in the range of 2.4 to 4.9ng/dl (66 to 136pmol/l) per mU/l.14 Our patient had a two ARR greater than 136pmol/l per mUI/l. The recent clinical practice guidelines for case detection, diagnosis, and treatment of patients with PA recommend that patients with positive ARR undergo any of four suppression tests to confirm or exclude the diagnosis of PA.14This implies that PA is defined as a non-suppressible aldosterone excess. The four suppression tests respectively use oral sodium loading, oral fludrocortisones, oral captopril, or saline infusion to suppress aldosterone secretion.9 Oral sodium load test includes the determination of sodium and aldosterone concentration in 24-hour urine, after 3 days of increased salt intake. Rates of aldosterone >12mg and sodium>200mmols are diagnostic of PA.6 This test confirmed the presence of PA in our patient (Aldosterone: 27.3mg/24h, Sodium 260mmols/24h). We did not proceed to saline infusion test (intravenous infusion of 2 liters of saline in four hours leads to a PAC rate over 277pmol/l if PA is present), oral captopril test and fludrocortisones test. The fludrocortisones suppression test is rarely performed in most centers nowadays.6 Distinction of disease subtypes is based on CT, adrenal vein catheterization with venous sampling and genetic screening for the familial types of the disease.14 In our patient, abdominal CT demonstrated a 28x21 mm hypo-dense mass (1UH of density), not enhanced by the contrast product, over the left adrenal gland. Unenhanced attenuation values on CT<10 UH indicated adenoma, as carcinoma present with values over 10 HU.15 The presence of an adenoma in patients with PA suggests the presence of an APA, but it cannot exclude the combination of a non-secreting adenoma and idiopathic PA.14,16 For distinction of the disease subtypes, a more practical approach use for the adrenal vein sampling (AVS) as recommended by Young.6 which is based on patient preferences, age, adrenal morphologic appearance on CT, clinical comorbid conditions, and clinical probability for finding an APA. For patients younger than 40 years, in whom a solitary adenoma is >1cm with normal contralateral adrenal gland, a unilateral adrenalectomy may be done without AVS and in the absence of comorbid conditions. Therefore, the AVS was bypass in our case based on these criteria.
Concerning therapeutic approach, the goal is the prevent the morbidity and mortality associated with hypertension, hypokalemia and cardiovascular damage.17 The cause of the PA helps to determine the appropriate treatment. Normalization of blood pressure should not be the only goal in managing a patient who has PA. In addition to the kidney and the colon, mineral corticoid receptors occur in the heart, brain and blood vessels. Excessive secretion of aldosterone is associated with increased risk of cardiovascular disease and morbidity. Therefore, normalization of circulating aldosterone or mineral corticoid receptor blockade should be part of the management plan for all patients with PA.17 Unilateral laparoscopic adrenalectomy is the preference treatment for patients with APA, and bilateral hyperplasia should be treated with mineral corticoid antagonists: spironolactone or eplerenone. Cardiovascular morbidity caused by aldosterone excess can be decreased by either unilateral adrenalectomy or mineral corticoid antagonist. Our patient was treated with laparoscopic left adrenalectomy after using spironolactone 100mg/day and nicardipine 150mg/day for one month preoperatively, leading to correction of hypokalemia and optimal control of blood pressure.
PA is the commonest cause of secondary arterial hypertension. APA is the second most important cause of PA and represents one of the few curable causes of secondary hypertension. Patients with PA have higher cardiovascular morbidity and mortality. The identification of the underlying disease of PA is substantial, since unilateral disorders are treated with adrenalectomy, while bilateral disorders are treated conservatively by aldosterone receptor antagonists.
None
Authors declare no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.