eISSN: 2577-8285


Research Article Volume 3 Issue 1
1Department of Human Performance and Leisure Studies, North Carolina A & T State University, USA
2Department of Kinesiology, University of Mary, USA
3Heart Institute Bnai-Zion Haifa Medical Center, Technion, Haifa, Israel
4Life Sciences Department, The Zinman College of Physical Education at the Wingate Collage, Israel
Correspondence: Moran Sciamama-Saghiv, Associate Professor of Clinical Exercise Physiology Chair, Department of Human Performance and Leisure Studies, College of Health and Human Sciences NC A&T State University Corbett HPER Center, Suite 215, room 216 1601 East Market Street/John Mitchell Drive Greensboro, North Carolina 27411, USA, Tel 336.285.3560, Fax 336.334.7258
Received: January 17, 2019 | Published: February 4, 2019
Citation: Saghiv M, Welch L, Goldhammer E, et al. The effects of partial sleep deprivation and the sub-maximal NDKS exercise testing protocol on S-Klotho and hemodynamic responses in. Sleep Med Dis Int J. 2019;3(1):17-23. DOI: 10.15406/smdij.2019.03.00061
Purpose: The purpose of the study was to examine the influences of partial sleep deprivation on young healthy trained women’s responses to the sub-maximal NDKS exercise testing protocol.
Methods: 32females 20.4±1.67 years of age, volunteered to participate in this study. Subjects underwent two sub-maximal NDKS exercise tests, once after 7-8 hours of sleep, and once after staying awake for 20hours. S-Klotho, blood pressure, heart rate, fasting blood glucose, and lactate were obtained at baseline, immediate-post, and post a dynamic recovery of 15minutes.
Results: S-Klotho at baseline was 422.09±32.62(pg·mL-¹). Significant differences were found between conditions regarding immediate-post S-Klotho concentration (547.18±51.75 vs 501.15±73.33 (pg·mL-¹), respectively, p ≤ 0.01). Significant differences were found between conditions regarding post-recovery S-Klotho concentration (443.31±35.66 vs 471.48±52.84(pg·mL-¹), respectively, p ≤ 0.01).
Conclusion: While not all comparisons for data obtained were significantly different, subjects had impaired responses while partially sleep deprived. In addition, partial sleep deprivation influenced S-Klotho concentrations for the worst while compared to non-sleep deprivation.
Keywords: women, partial sleep deprivation, S-Klotho, NDKS, hemodynamic responses, exercise testing, blood pressure, glucose, lactate, heart rate
It has long been known that different extents of sleep deprivation (partial and/or full) have negative implications on function, hemodynamic, and physiological responses.1–6 The influences of sleep deprivation may in some cases, be sex-specific (male vs female). Such influences may be characterized by neurologic, endocrine, physiologic-metabolic, and behavioral changes, often for worse.7–9 Prior studies pertaining to the influences of sleep deprivation of different extent clearly indicate that while sleep deprived, multiple aspects of function, across several systems are influenced for the worse. Such influences may include endothelial dysfunction, hypertension, decreased brain activity, cardiac dysfunction, heart rate variability, arrhythmias, blood pressure, renal function, and more.10–13 On the other hand, exercise and adaptation to exercise of various exercise modalities such as aerobic training, and resistance training have been proven to negate part and/or all of the adverse effects of sleep deprivation in humans and rats.14–17
S-Klotho is an enzyme that in humans is encoded by the KL gene.18 S-klotho is a type I membrane protein first documented in 1997.19 The protein is associated with the degenerative process, acceleration of aging, bone lose, and alcohol consumption.20–24 Furthermore, S-Klotho is a role-player in determining the sensitivity to insulin, mediating the binding of FGF19, FGF20, and FGR23 to their receptors as part of the growth process at the cellular level.23 In addition, S-Klotho is suggested as a cardiovascular system “protector” via means of endothelium-derived NO production,25,26 and has been proven to affect cellular calcium homeostasis via increased expression of TRPV5, and decreased TRPC6; and increases membrane expression of inward rectifier ROMK. In mice, under-expression causes hyper-vitaminosis of vitamin D; altered mineral-ion homeostasis resulting in accelerated aging; a syndrome of accelerated aging; arteriosclerosis; impaired endothelium-dependent vasodilation; and impaired angiogenesis.23;27–30
In mice, over-expression may prolong life span by 19-31%.In humans, exercise modalities influence Klotho gene expression both epigenetically and positively.31 Prior studies show improvements in aerobic capacity, exercise tolerance, and power output influence the levels of circulating S-Klotho for the better.32,33 To the best of the authors’ knowledge, no prior published data exists as to the influence of partial (less than 40hours of lack of sleep) and/or full sleep deprivation (more than 40hours of lack of sleep) on S-Klotho in women and/or during and after an exercise test of any sort. Thus, the aim of this study was to investigate the influences of partial sleep deprivation (20hours of lack of sleep) on S-Klotho concentration (pg·mL-¹). In addition, as part of the study, the influence of partial sleep deprivation on heart rate (bpm), blood pressure (mmHg), fasting blood glucose (mg∙dL-1), lactate (mmol∙L-1), oxygen saturation (O2sat, %) and rate of perceived exertion (RPE, scale) before and after a Nustad, Dressler, Kobes, Sagiv (NDKS) sub-maximal exercise testing protocol in young, healthy, and trained women.
Overview, subjects, recruitment and enrollment
After achieving Institutional Review Board (IRB) approval to conduct this study, subjects were asked to participate in the study. Thirty-two women, 20.4±1.67 years of age, volunteered to participate in this study. All participants were healthy and trained individuals. Subjects visited with the researcher 3-4 times. Upon agreeing to participate, subjects received an informed consent form via email and/or hard-copy, read the form, and indicated in a clear manor they have fully understood its content, prior to signing the form. The subjects were given as many opportunities as needed and/or wanted to ask questions pertaining to the study’s purpose, protocol, etc. Subjects then filled-out a health history questionnaire (HHQ) and signed the HHQ. The information in the HHQ and follow-up questions (if raised) were used to determine rather or not subjects were to be included or excluded from the study. Prospective subjects were excluded if they had diagnosed musculoskeletal injury, cardiovascular disease, pulmonary disease, mental health problems, endocrine instability, and/or were treated with medication for any reason.
Baseline measurements
Following inclusion in the study (as part of the first visit with the researchers), the subjects’ height, weight, heart rate, blood pressure, O2sat, fasting blood glucose, blood lactate, and a 5mL blood sample (from the median cubital vein) were obtained, recorded on file and/or labeled. Prior to leaving the lab, subjects were randomly assigned to their two sub-maximal NDKS exercise tests. Using a table of random numbers, half of the subjects were assigned to be tested non-partially sleep deprived first (NPSD), and the other half, scheduled to be tested partially sleep deprived first (PSD). Subject was allowed to leave the lab in the absence of adverse reactions and/or return to baseline values.
Prior to exercise testing sessions
Subjects were instructed to completely avoid alcohol consumption of any sort, moderate/intensive exercise of any sort, caffeine, use of any over-the-counter medications, and/or stressful situations at least 8hours prior to data collection. Subjects self-reported their compliance with these instructions.
Exercise testing (NPSD or PSD)
Subjects tested while partially sleep deprived, reported to the lab in 30-minute increments starting at 2am (up to six subjects were tested per data collection session; two at a time, and no more than three pairs altogether). Subjects were instructed to be completely awake from 6am the prior day. Subjects were contacted via email, text message, and/or phone call the day before testing to assure compliance with the instructions. Subjects tested while non-partially sleep deprived, reported to the lab in 30-minute increments starting at 6am (up to six subjects were tested per data collection session; two at a time, and no more than three pairs altogether). Subjects were instructed to sleep 7.5-8 hours prior to testing. Subjects contacted via email, text message, and/or phone call the day before testing to assure compliance with the instructions. Upon arrival at the lab, the subjects were asked if they have complied with the instructions (one after the other). Subjects non-compliant with the instructions were rescheduled; all subjects were tested twice within a period of no more than two weeks in between test, and for the vast majority of cases, within a week apart. Subjects compliant and without signs of a health condition (cold, influenza, etc.) were seated for five minutes, doing nothing. Afterwards, heart rate, and blood pressure were obtained and compared to the subjects’ baseline values. The subjects then continued to a warm up, followed by a NDKS sub-maximal exercise test, and dynamic recovery following the test itself. The exercise test was terminated once the subjects achieved 85% of her age-estimated maximal heart rate according to the equation [HR85% = 0.85×(220-age)] or if the subject wished so for any reason. Heart rate, blood pressure, RPE (6-20 scale), and O2sat were obtained every other minute during the exercise test. S-Klotho was obtained after subjects have undergone a dynamic recovery that included waking at 1.7 miles per hour at 0% grade for the duration of fifteen minutes.
Samples and measurements
5mL blood samples were obtain (according to universal precautions for blood borne pathogens) from the median cubital vein and stored in BD Vacutainer™ Venous Blood Collection Tubes: SST™ Serum Separation Tubes with Conventional Stopperuntil analyzed; samples were then refrigerated. Subject without adverse reactions and upon return to the close vicinity of baseline values were allowed to leave the lab. Blood samples were analyzed via Soluble Klotho (Human serum) ELISA Kit SK00708-08 (Adipo Bioscience, Inc.), with a Standard range of 313-20000 pg∙mL-1; Sensitivity of 80 pg∙mL-1; Intra-CV of 4-6%; and Inter-CV of 8-10%. Blood pressure was obtained utilizing an Omron sphygmomanometer; heart rate via Polar heart rate monitor and strap, oxygen saturation was obtained via AccuMed CMS-50D Pulse Oximeter Finger Pulse Blood Oxygen SpO2 Monitor. Lactate and glucose (mg∙dL-1) were obtained via Point-of-Care Testing using a Reagent i-STAT CG4+ Blood Gases Blood Analyzer and a Roche Diagnostics Accutrend Glucose Control 2 Levels respectively. The treadmill used in this study was a NordicTrack C 990 Treadmill.
Statistical analysis
SPSS 23 for Windows was used to analyze the data, Comparisons between conditions (partial sleep deprivation vs non-partial sleep deprivation) were conducted via One-way repeated measures ANOVA and the Tukey post hoc test. Significance was set at p ≤ 0.05. Results are presented as means ± standard deviation.
In general
All subjects participated in this study without any prolonged adverse reactions. Some subjects had a temporary reaction to their blood being drawn (dizziness, elevated heart rate, hypertension, hypotension, and in three cases subjects reacted with syncope). All subjects left the lab without any adverse clinical signs.
Baseline measures
Presented in Table 1 are data for height, weight, BMI, heart rate, blood pressure, glucose, lactate, and oxygen saturation.
Variable |
Mean±SD |
N |
32 |
Height (cm) |
167±5.16 |
Weight (Kg) |
69.55±7.03 |
BMI (Kg∙m-2) |
24.94±0.56 |
HR (bpm) |
71.3±4.04 |
SBP (mmHg) |
116.6±7.11 |
DBP (mmHg) |
67.32±6.31 |
Glucose (mg∙dL-1) |
96.25±15.21 |
Lactate (mmol∙L-1) |
1.7±1.33 |
O2sat (%) |
98.06±0.79 |
Table 1 Baseline values for height, weight, BMI, heart rate, blood pressure, glucose, lactate, and O2sat (mean±SD)
BMI, Body Mass Index; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; O2sat, Oxygen saturation; cm, centimeters; Kg, kilograms; m, meter; bpm, beats per minute; mmHg, millimeter mercury; dL, deciliter; L, liter; mmol, milimole; %, percentage.
S-Klotho
S-Klotho concentrations were significantly higher (p ≤ 0.05) immediate-post compared to at rest and post dynamic recovery. No significant differences (p > 0.05) were found between groups post dynamic recovery. S-Klotho levels immediate-post was significantly higher for the NPSD group compared to that of the PSD group (p ≤ 0.05). Dynamics of S-Klotho concentration are presented in Table 2 and Figure 1 below.
Condition |
Variable |
Baseline |
Immediate-post |
Post dynamic recovery |
NPSD |
Average |
422.09 |
547.18†α |
433.31†‡ |
SD |
32.62 |
51.75 |
35.66 |
|
PSD |
Average |
N/A |
501.15† |
471.48† |
SD |
N/A |
73.33 |
52.84 |
Table 2 S-Klotho (pg·mL-¹) concentrations according to condition and stage of exercise testing (mean±SD).
NPSD, non-sleep deprived; PSD, partially sleep deprived; SD, standard deviation; †, significant difference compared to baseline under the same condition; ‡, significant difference compared to immediate post under the same condition; α, significant difference between conditions for the same timing of obtaining.
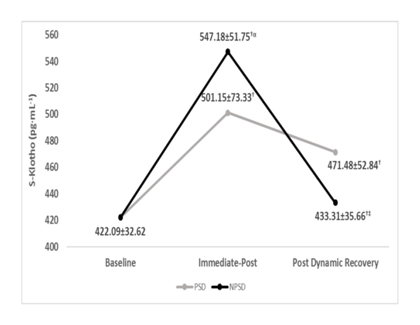
Figure 1 S-klotho (pg·mL-¹) concentrations according to condition (mean±SD)
NPSD, non-sleep deprived; PSD, partially sleep deprived; SD, standard deviation; †, significant difference compared to baseline under the same condition; ‡, significant difference compared to immediate post under the same condition; α, significant difference between conditions for the same timing of obtaining.
Heart rate
Significant differences (p≤0.05) were found between conditions for the heart rate at the start of the exercise test, and stages 3,4 & 6.While being partially sleep deprived, heart rate during the fifth stage was non-significantly higher (p>0.05) compared to that of stage 4. Presented in Figure 2 are changes in heart rate according to the stage of the NDKS sub-maximal test.
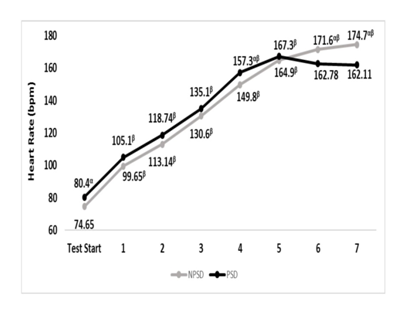
Figure 2 Changes in heart rate (bpm) according to condition, during the sub-maximal NDKS exercise test (mean).
Bpm, beats per minute; NPSD, non-sleep deprived; PSD, partially sleep deprived; α, significant difference between conditions; β, significant difference from the measurement of a stage prior in the same condition.
Systolic blood pressure
Systolic blood pressure was significantly higher (p≤0.05) while partially sleep deprived all but during stages 4 & 5. For both conditions, all comparisons were significantly different (p≤0.05) between stages within the condition. Changes in systolic blood pressure according to the stage of the NDKS sub-maximal test are presented in Figure 3.
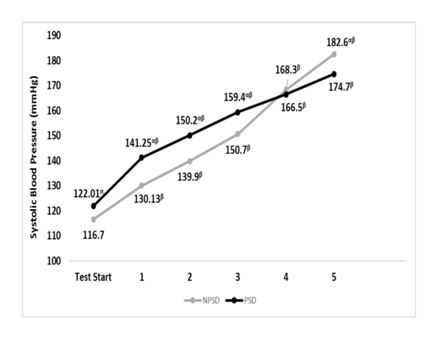
Figure 3 Changes in systolic blood pressure (mmHg) according to condition, during the sub-maximal NDKS exercise test (mean).
SBP, systolic blood pressure; RSBP, resting systolic blood pressure; mmHg, millimeters of Mercury; NPSD, non-sleep deprived; PSD, partially sleep deprived; blood pressure not measured while running; α, significant difference between conditions; β, significant difference from the measurement of a stage prior in the same condition.
Diastolic blood pressure
Diastolic blood pressure was within a very narrow range for both conditions and during all stages. None of the possible comparisons was significant (p>0.05). Changes in diastolic blood pressure according to the stage of the NDKS sub-maximal test are presented in Figure 4.
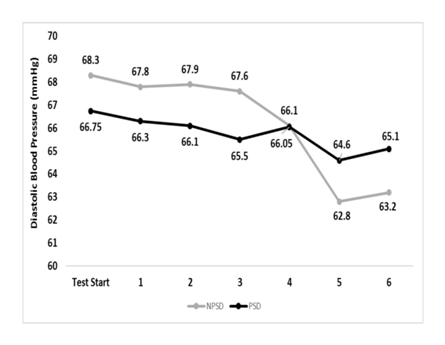
Figure 4 Changes in diastolic blood pressure (mmHg) according to condition, during the sub-maximal NDKS exercise test (mean).
DBP, diastolic blood pressure; RDBP, resting diastolic blood pressure; mmHg, millimeters of Mercury; NPSD, non-sleep deprived; PSD, partially sleep deprived; blood pressure not measured while running; α, significant difference between conditions; β, significant difference from the measurement of a stage prior in the same condition.
RPE
A significant difference (p≤0.05) between conditions was found during the last stage. Differences within the same condition and in between stages were all significant (p≤0.05), all but between stages 1 & 2 while non-sleep deprived. Changes in rate of perceived exertion according to the stage of the NDKS sub-maximal test are presented in Figure 5.
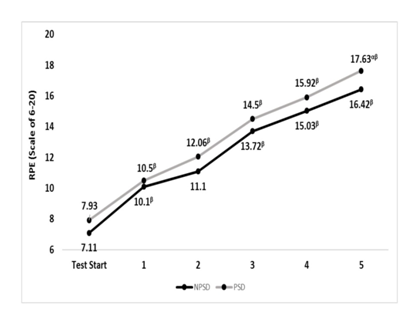
Figure 5 Changes in rate of perceived exertion (RPE; Scale) according to condition (mean).
RPE, rate or perceived exertion; NPSD, non-sleep deprived; PSD, partially sleep deprived; α, significant difference between conditions; β, significant difference from the measurement of a stage prior in the same condition.
Glucose
Immediate-post glucose decreased in both conditions significantly (p≤0.05) compared to baseline levels, then continued to increase in a non-significant (p>0.05) extent until after the dynamic recovery. All comparisons between conditions were non-significant (p>0.05). Changes in fasting blood glucose according to the stage of the NDKS sub-maximal test are presented in Figure 6.
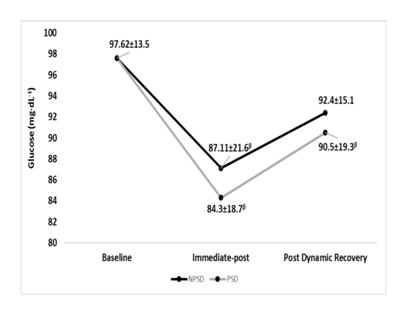
Figure 6 Changes in Glucose (mg∙dL-1) according to condition (mean).
mg/dL, milligrams per deciliter; NPSD, non-sleep deprived; PSD, partially sleep deprived; α, significant difference between conditions; β, significant difference from the measurement of a stage prior in the same condition.
Lactate
Lactate has increased significantly (p≤0.05) from rest to immediate-post for both conditions, then has significantly (p≤0.05) decreased for both conditions. Significant differences (p≤0.05) were found between conditions all but at baseline. Changes in blood lactate according to the stage of the NDKS sub-maximal test are presented in Figure 7.
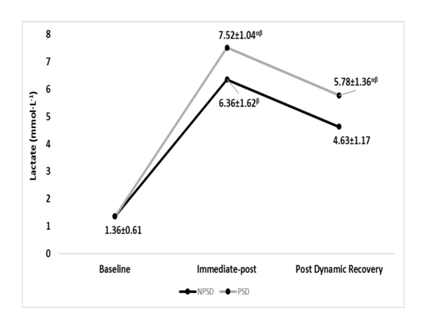
Figure 7 Changes in Lactate (mmol∙L-1) according to condition (mean).
mmol/L, millimole per liter; NPSD, non-sleep deprived; PSD, partially sleep deprived; α, significant difference between conditions; β, significant difference from the measurement of a stage prior in the same condition.
Oxygen saturation
Oxygen saturation levels were significantly different (p≤0.05) within the conditions while comparing the values of stage five to those of the start of the test and stage 1. No significant differences (p>0.05) were found between conditions. Changes in oxygen saturation according to the stage of the NDKS sub-maximal test are presented in Figure 8.
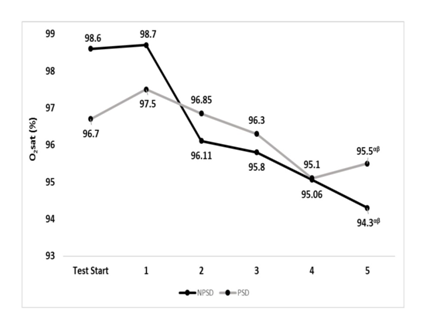
Figure 8 Changes in Oxygen saturation (O2sat; %) according to condition (mean).
O2sat, oxygen saturation (%); Stg, stage number of the sub-maximal NDKS exercise test; NPSD, non-sleep deprived; PSD, partially sleep deprived; α, significant difference between stage 5 and test start; β, significant difference between stage 5 and stage 1.
This study is a first of a kind in regards to partial sleep deprivation and its influences on S-Klotho levels in women before and after sub-maximal exercise testing. The researchers are unaware of published data pertain to the topic. With that said, S-Klotho is a biomarker of aging and anti-aging alike, dependent on the extent of gene expression.32,33 Thus, this study starts to shade light on the connection between partial sleep deprivation and aging/anti-aging represented via S-Klotho concentration under certain conditions. Sleep deprivation (acute and/or chronic) is well documented as an influence of function and performance in both healthy and clinical populations, across sex and age to different extents.1–17 The present study demonstrates a clear and significant influence of partial sleep deprivation on S-Klotho responses to the sub-maximal NDKS exercise test in young, healthy, and trained women, while compared to being non-sleep deprived. The data of this study suggest that partial sleep deprivation of 20hours, is a strong enough stressor, resulting in lower levels of S-Klotho immediate-post, and post-dynamic recovery, while compared to those of the non-sleep deprived state. Partially sleep-deprived, and from baseline to 85% of age-estimated heart rate maximum, subject were unable to elevate S-Klotho levels, although the same subjects were able to do so while non-sleep deprived Appendix A. Though the design of the study does not allow proving the reason for this dynamic, it is the researchers’ suggestion that the inability to elevate S-Klotho as much as while non-sleep deprived represents animpaired reaction, induced by fatigue.
Minutes |
Workload |
Comments |
Warm-up |
2.3mph × 0% grade |
Measurements taken |
1 |
3.1mph × 5% |
Measurements taken |
2 |
3.7mph × 5% |
|
3 |
3.7mph × 6% |
Measurements taken |
4 |
4.3mph × 6% |
|
5 |
4.3mph × 7% |
Measurements taken |
6 |
5.0mph × 7% |
|
7 |
5.0mph × 8% |
Measurements taken |
8 |
5.6mph × 8% |
|
9 |
5.6mph × 9% |
Measurements taken |
10 |
6.2mph × 9% |
|
11 |
6.2mph × 10% |
Measurements taken |
12 |
6.8mph × 10% |
|
13 |
6.8mph × 11% |
Measurements taken |
14 |
7.4mph × 11% |
|
15 |
7.4mph × 12% |
Measurements taken |
16 |
8.0mph × 12% |
|
17 |
8.0mph × 13% |
Measurements taken |
18 |
8.6mph × 13% |
|
19 |
8.6mph × 14% |
Measurements taken |
20 |
9.2mph × 14% |
|
21 |
9.2mph × 15% |
Measurements taken |
22 |
9.8mph × 15% |
|
23 |
9.8mph × 16% |
Measurements taken |
24 |
10.4mph × 16% |
|
Appendix A The NDKS aerobic exercise test
Proper sleep has been indicated as an anti-oxidative factor.34,35 On the other hand, exercise induces the creation of reactive oxygen species (ROSs).36–38 Thus, it is possible that the need to elevate S-Klotho during exercise is partially in order to assist with the need to battle ROSs. The impaired response is further apparent at post-dynamic recovery, whereas the level during non-sleep deprivation return almost completely to baseline, most likely indicating the ability to counteract ROSs better than while partially sleep deprived. Baseline heart rate values for this group of participants were within the range of previously reported findings for age, training status, and sex matching subjects.39,40 Both resting systolic and diastolic blood pressure values of the subjects in this study were within normal and healthy ranges.41 Subjects’ average resting fasting blood glucose was within the normal glucose range considered healthy (<100 mg∙dL-1).42 Lactate concentrations were within the previously reported range for sex, age, and training status.43 Oxygen saturation measured via pulse-oximeter showed that subjects’ oxygenation levels were adequate and contradicted a diagnosis of hypoxemia.44 These results are similar, yet lower than those reported whereas oxygen saturation was 98.53±0.52%.45 Subjects of this study have been tested in a sub-maximal treadmill exercise test, and have achieved greater heart rate peak values than previously reposted. Significant influences of sleep deprivation on peak heart rate in young, healthy, female students undergoing a sub-maximal treadmill test were previously reported while comparing men and women under both conditions. Heart rate peak under partial sleep deprivation was significantly lower.1
Blood pressure peak post-exercise was reported as significantly different between conditions of sleep deprivation and non-sleep deprivation; All additional variables were not significantly different. Results found in both studies are similar. The findings of this study as they pertain to RPE contradict prior findings whereas sleep deprivation resulted in elevated RPE. An interesting finding of these studies is the fact that physiological measures where not significantly affected while RPE increased, perhaps hinting that the cognitive and psychological component outweighs the physiological aspect.2,46 While RPE was higher while partially sleep deprived, the differences were not great enough to induce a statistical significance. Similar non-significant findings regarding RPE were reported previously.46 Recent data suggests that Oxygen saturation is not significantly altered due to partial sleep deprivation during exercise testing. Such findings were reported while investigating the effects of two types of partial sleep deprivation (PSD) on biomarkers of muscle and cardiac injuries in response to acute intermittent exercise in professional athletes.4 These findings align perfectly with the findings of this study. Prior studies have shown that partial sleep deprivation has no significant effect on Glucose, yet 48hours of sleep deprivation (full sleep deprivation) results in a 40% greater insulin resistance level.5 While this study did not investigate the influences of partial sleep deprivation on insulin and/or insulin resistance, the findings of this study align with those of prior studies as to the non-significant effects of partial sleep deprivation on fasting blood glucose levels.5 Very few data exist regarding the influences of sleep deprivation on lactate and/or lactic Acid. The findings of this study contradict a previous report whereas partial sleep deprivation caused a significant increase in lactate.6
The data of this study suggest that partial sleep deprivation is a strong enough stimulus affecting S-Klotho concentration in response to the sub-maximal NDKS aerobic exercise testing protocol. Our results further support the possibility of an impaired ability to elevate S-Klotho as a result of sub-maximal exercise, and impaired ability to return to baseline levels post-dynamic recovery. The results of this study fairly align with the majority of existing data pertaining to the lack of significant influences of partial sleep deprivation on physiological variables, all but RPE.
None.
The authors declare that there is no conflict of interest.

©2019 Saghiv, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.