eISSN: 2577-8285


Short Communication Volume 4 Issue 3
1Department of Pulmonary Diseases and Clinical Allergology, University of Turku, Finland
2Division of Medicine, Department of Pulmonary Diseases, Turku University Hospital, Finland
3Auria Biobank, University of Turku and Turku University Hospital, Finland
4Department of Anaesthesiology, University of Turku, Finland
5Department of Paediatrics and Adolescent Medicine, Turku University Hospital and University of Turku, Finland
6Department of Hospital Hygiene and Infection Control, Turku University Hospital (TYKS), Finland
7Department of Infectious Diseases, Turku University Hospital, University of Turku, Finland
Correspondence: Thijs Feuth, PhD, Turku University Hospital, Department of Pulmonary Diseases and Allergology, Kiinamyllynkatu 4-8, 20410, Turku, Finland, Tel +358-45-1564939
Received: August 29, 2020 | Published: October 22, 2020
Citation: Feuth T, Saaresranta T, Karlsson A, et al. Is sleep apnea a risk factor for Covid-19? findings from a retrospective cohort study. Sleep Med Dis Int J. 2020;4(3):61-65. DOI: 10.15406/smdij.2020.04.00075
Background: In the early phase of the coronavirus disease-19 (Covid-19) pandemic, Southwest Finland remained relatively spared. By the 3rd of May 2020, a total of 28 patients have been admitted to the Turku University Hospital. We explore baseline characteristics in order to identify risk for severe disease and critical care admission.
Methods: For this retrospective cohort study, data were derived from hospital records. Basic descriptive statistics were used to characterise patients, including medians, percentiles and frequencies. Differences were tested with Mann Whitney U-test and Pearson’s chi-square test.
Results: Pre-existent obstructive sleep apnea (OSA) was present in 29% of patients admitted in the hospital for Covid-19. Overall, other findings on admission were comparable with those reported elsewhere. C-reactive protein and procalcitonin were higher in patients who were eventually transferred to critical care in comparison to in those who were not (median CRP 187 mg/L versus 52 mg/L, p<0.005 and median PCT 0.46 versus 0.12, p=0.047).
Conclusion: OSA was pre-existent in a disproportional large group of patients, which suggests that it is an important risk factor for severe Covid-19. Furthermore, we identified high CRP, PCT and possibly native oxygen saturation as useful clinical measures to identify patients at risk for critical care.
Keywords: Covid-19, sleep apnea, viral infection, respiratory infections
AHI, apnea-hypopnea index; APAP, automatic positive airway pressure; BMI, body mass index; Covid-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; CRP, C-reactive protein; CXR, chest X-ray; ESS, epworth sleepiness scale; ICU, intensive care unit; MAD, mandibular advancement device; ODI3, 3% oxygen desaturation index; OSA, obstructive sleep apnea; pCO2, partial pressure of carbon dioxide; PCT, procalcitonin; RAAS, renin angiotensin aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SpO2, peripheral capillary oxygen saturation
As the newly discovered Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is rapidly spreading around the world, Finland outside its capital Helsinki remains relatively spared in the early phase of the coronavirus disease-19 (Covid-19) pandemic. On the 3rd of May 2020, WHO reported 3,349,786 cases worldwide, of which 1,518,895 in Europe alone.1 By then, Southwest Finland, home to 479,234 people, had a reported incidence of Covid-19 of 263 cases. Of them, 28 patients had been admitted to the Turku University Hospital. In the first phase of the outbreak, cough and fever were the most reported symptoms.2 Advanced age, cardiovascular diseases and hypertension were among the first risk factors for severe disease and mortality to be recognized.3,4 In addition, obesity predisposes to severe Covid-19 requiring admission to critical care.5 Several mechanisms have been proposed to explain the link between obesity, cardiovascular disease and Covid-19, including low-grade chronic inflammation, chronic hypoxemia and oxidative stress and involvement of the renin angiotensin aldosterone system (RAAS) linked to expression of ACE2, the cellular receptor of SARS-CoV-2. However, the contribution of these mechanisms to Covid-19 pathology remain to be resolved.6–9 In this article, we report on the cases admitted to the Turku University Hospital, Finland, in the early phase of the pandemic. A post-hoc analysis was performed to investigate whether obstructive sleep apnea (OSA) is a potential risk factor for severe COVID infection.
In April 2020, we started an observational database to systematically collect clinical data of all Covid-19 patients admitted in the University Hospital of Turku. In this report, we document on all cases with positive PCR for SARS-CoV-2 who were admitted by May 3rd 2020. Data cut-off was the 28th of May 2020. Baseline clinical findings are first clinical measurements as reported upon admission at the emergency room. In cases with no newly measured body weight or height measured during admission, older values were retracted from the patient files if available. Baseline oxygen saturation is first measurement at presentation, before start of supplemental oxygen. Baseline laboratory tests were taken directly on admission or on the next weekday. For the sub-analysis of OSA patients, baseline sleep apnea data was retracted from patient files and from the ResMed CPAP-telemonitoringsystem.OSA was defined as apnea-hypopnea index (AHI)>5/h. The indication for treatment was AHI >15/h or AHI>5/h with symptoms markedly affecting the quality of life or work ability.
In all patients, SARS-CoV-2 was tested by polymerase chain reaction (PCR).
Statistics
For descriptive statistics, median and 25th to 75th percentiles and percentages in case of frequencies were reported. Differences in continuous variables between ICU and non-ICU patients were tested with the Mann-Whitney U test. Pearson’s chi-squared test was used to test for differences in frequencies. Correlations were tested with 1-tailed Spearman correlation efficient.
Ethics statement
According to the Finnish Medical Research Act (488/1999, sections 1-3), non-interventional studies do not require separate approval of the ethics committee. Approval for this study was obtained from the Turku University Hospital Clinical Research Centre, who did not require ethics committee approval or informed consent. The research was conducted according to the principles of the World Medical Association Declaration of Helsinki.
The first covid-19 patient was admitted to the Turku University Hospital, Finland, on March 6th 2020. By May 3rd 2020, a total of 28 patients had been admitted. By then, a total of 278 patients were diagnosed with Covid-19 by public services out of a total population of 479,234 people. Baseline clinical findings are summarized in Table 1. Of note, female patients made up to 46% of admitted cases. The cohort includes only 1 minor of 15 years old. A total of 7 patients (25%) were treated on ICU. By the 10th of May, 2 patients included in this study were still admitted in the hospital, with 1 on ICU. Median length of stay in hospital was 9.5 days, and median length of stay in ICU was 19 days. Fifteen patients (54%) could be discharged to home, 9 (32%) were discharged to a regional health centre. By May 10th, 2 patients had died in hospital and 1 after referral to a regional health centre. Of patients of whom smoking status was known, 11% were current smokers and 29% ex-smokers. Median blood pressure (133/77 mmHg) and heart rate (84/min) were in the normal range, as were hemoglobin (135 g/L), thrombocytes (234 e9/L), leukocytes (5.7 e9/L), and creatinine (79 µmol/L). Fever (median 38.0°C), hypoxemia (median SpO2 92%), lymphocytopenia (median 1.0x10e9/L), increased C-reactive protein (CRP, median 90 mg/L) and increased procalcitonin (PCT, median 0.13 µg/L) were common. Chest X-ray (CXR) showed bilateral opacities in 68% of cases and unilateral opacities in 16%. Common pre-existent conditions included hypertension in 43% of cases, obesity (in 37%), OSA (29%), diabetes mellitus (25%), asthma (14%), COPD (7%) and active malignant disease (11%). Baseline characteristics are summarized in Table 1. Baseline characteristics were compared between patients who were later on admitted to ICU (n=7) and those who were not (non-ICU, n=21). Baseline CRP (median 187 mg/L) and PCT (median 0.46 µg/L) were significantly higher among those who were later on transferred to ICU compared to those who were not (median CRP 52 mg/L, p<0.005 and median PCT 0.12, p=0.047). In addition, a trend towards lower native SpO2 on admission was observed in ICU-patients (87%) in comparison with non-ICU patients (93%, p=0.09). All ICU-patients had bilateral opacities in CXR on admission to the hospital Table 1 & Figure 1.
|
All patients |
25th-75th |
ICU-patients |
non-ICU |
p-value | |
|
Gender (% females) |
46 |
43 |
1 |
||
|
Age (years) |
56 |
47–72 |
55 |
58 |
0.64 |
|
Weight (kg) |
82 |
75–101 |
83 |
81 |
0.435 |
|
BMI (kg/m2) |
28.4 |
24.6–31.3 |
29.4 |
28.1 |
0.893 |
|
Smoking history |
0.177 |
||||
|
Non-smoker |
17/28 (61%) |
4/7 (57%) |
13/21 (62%) |
||
|
Ex-smoker |
8/28 (29%) |
1/7 (14%) |
7/21 (33%) |
||
|
Current smoker |
3/28 (11%) |
2/7 (29%) |
1/21 (5%) |
||
|
Systolic blood pressure (mmHg) |
133 |
112–151 |
147 |
132 |
0.756 |
|
Diastolic blood pressure (mmHg) |
77 |
67–83 |
78 |
76 |
0.756 |
|
Heart rate (bpm) |
84 |
70–94 |
93 |
82 |
0.629 |
|
Native oxygen saturation (%) |
92 |
87.3–96.8 |
87 |
93 |
0.09 |
|
Temperature (°C) |
38 |
37.0–38.6 |
38 |
38 |
0.745 |
|
Hemoglobin (g/L) |
135 |
122–145 |
143 |
133 |
0.717 |
|
Leukocytes xE9/L |
5.7 |
3.7–8.2 |
8.1 |
5.5 |
0.228 |
|
Lymphocytes xE9/L |
1 |
0.7–1.2 |
0.74 |
1.09 |
0.244 |
|
Trombocytes xE9/L |
234 |
164–262 |
247 |
229 |
0.646 |
|
C-reactive protein (mg/L) |
90 |
31-142 |
187 |
52 |
<0.005 |
|
Procalcitonin (μg/L) |
0.13 |
0.06–0.45 |
0.46 |
0.12 |
0.047 |
|
D-dimer (mg/L) |
0.4 |
0.2–1.5 |
0.5 |
0.4 |
0.757 |
|
Creatinine (μmol/L) |
79 |
55–132 |
79 |
74 |
0.685 |
|
Chest X-ray finding |
0.102 |
||||
|
Normal |
4/25 (16%) |
0/7 (0/7) |
4/18 (22%) |
||
|
Unilateral pneumonia |
4/25 (16%) |
0/7 (0%) |
4/18 (22%) |
||
|
Bilateral pneumonia |
17/25 (68%) |
7/7 (100%) |
10/18(56%) |
||
|
Pre-existing conditions |
|||||
|
Obesity |
10/27 (37%) |
3/7 (43%) |
7/20 (35%) |
0.711 |
|
|
Diabetes mellitus |
7/28 (25%) |
2/7 (29%) |
5/21 (24%) |
0.801 |
|
|
Obstructive sleep apnea |
8/28 (29%) |
3/7 (43%) |
5/21 (24%) |
0.334 |
|
|
Hypertension |
12/28 (43%) |
4/7 (57%) |
8/21 (38%) |
0.378 |
|
|
Asthma |
4/28 (14%) |
1/7 (14%) |
3/21 (14%) |
1 |
|
|
COPD |
2/28 (7%) |
0/7 (0%) |
2/21 (10%) |
0.397 |
|
|
Malignancy |
3/28 (11%) |
0/7 (0%) |
3/21 (14%) |
0.29 |
|
|
Pre-existing drug use |
|||||
|
ACE-inhibitor |
5/28 (18%) |
1/7 (14%) |
4/21 (19%) |
0.776 |
|
|
ARB |
4/28 (14%) |
2/7 (29%) |
2/21 (10%) |
0.212 |
|
|
Prednisolone |
5/28 (18%) |
2/7 (29%) |
3/21 (14%) |
0.393 |
|
Table 1 Baseline characteristics on admission to the hospital of all patients (n=28), those who were eventually admitted to the ICU and those who were not (non-ICU). Depicted are median values and 25th to 75th percentiles for continuous variables and percentages for frequencies
ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; IQR, interquartile range (25th to 75th percentile)
P-values of continuous variables are tested by Mann Whitney U-test and are not corrected for multiple testing and should not be used to infer definitive effects. P-values of frequencies are tested by Pearson Chi Square test
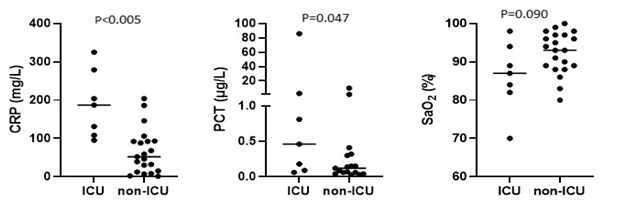
Figure 1 Baseline characteristics and admission to critical care.
CRP, C-reactive protein; PCT, procalcitonin; SpO2 native oxygen saturation (without supplemental oxygen)
CRP, procalcitonin and SpO2 on admission in patients who later on required admission to the ICU and in those who were treated on the conventional ward (non-ICU). Horizontal lines represent median values. P-values are tested with Mann Whitney U-test.
Native SpO2 was correlated with lymphocytes (r 0.37, p = 0.036) and was negatively correlated with age (r-0.48, p=0.005) and CRP (r -0.365, p=0.029) Figure 2. In the Hospital District of Southwest Finland, a total of 12,799 people (prevalence 2.7%) are treated with continuous positive airway pressure (CPAP) therapy and around 2,000 people with mandibular advancement device (MAD) for OSA. Thus, prevalence of hospital-treated OSA is around 3.1%. As prevalence of previously diagnosed OSA was disproportionally high in this cohort, we performed a post-hoc descriptive analysis of those patients. BMI>30kg/m2 was found in 75% of OSA patients. Median BMI was 38.0 kg/m2. Thus, the majority of OSA patients in this cohort were severely obese. Median time of diagnosis of OSA was 2.5 years before Covid-19 and at time of diagnosis, median AHI was 18/h. Median 3% oxygen desaturation index (ODI3) was 12/h. At time of diagnosis of OSA, average overnight SpO2 was 92.1% and minimum was 83.5% (median values) and morning pCO2 values were in the normal range in all cases (median 4.9 kPa). One patient was using MAD and the other 7 patients were CPAP-users (six using automatic positive airway pressure (APAP) device and one fixed pressure CPAP). CPAP adherence was good (median 7.5 hours) in those actually using the device (n=5), but two patients had discontinued at least a month before admission for Covid-19. Characteristics of OSA patients in our case series are summarized in Table 2.
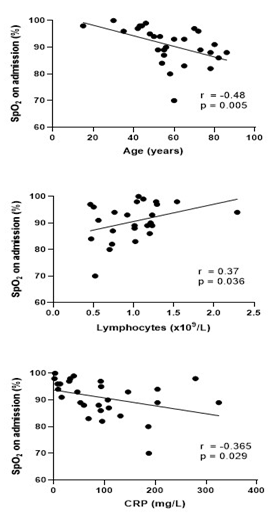
Figure 2 Correlations between native oxygen saturation with age, lymphocytes and CRP
SpO2 initial native oxygen saturation at presentation; CRP, C-reactive protein Correlations were tested with Spearman r correlation coefficient. Lines depict simple linear regression.
|
Median |
25th–75th percentile |
|
|
Diagnosis of sleep apnea(y)1 |
2.5 |
1,3–15 |
|
BMI (kg/m2)2 |
38 |
34.3–41.6 |
|
AHI (/h) |
18 |
9–24 |
|
ODI3 (/h) |
12 |
7.7–12.6 |
|
ESS |
3 |
1–8 |
|
Average night SpO2 (%) |
92.1 |
89.8–94.3 |
|
Minimum night SpO2 (%) |
83,5 |
81.0–88.0 |
|
pCO2 (kPa) |
4.9 |
4.6–5.2 |
|
Mode of treatment |
||
|
APAP |
6/8 (75%) |
|
|
Fixed CPAP |
1/8 (13%) |
|
|
MAD |
1/8 (13%) |
|
Table 2 Characteristics of pre-existent sleep apnea in patients with Covid-19
BMI, body mass index; AHI, apnea-hypopnea index; ODI3, 3% oxygen desaturation index; ESS, epworth sleepiness scale; APAP, automatic positive airway pressure; CPAP, continuous positive airway pressure; MAD, mandibular advancement device; SpO2, oxygen saturation (without supplemental oxygen); pCO2, partial pressure of carbon dioxide
1Time since diagnosis of sleep apnea before diagnosis of Covid-19. 2Most recent BMI
Most of our findings were generally consistent with reports of Covid-19 cases elsewhere.2,4,5 However, two of our findings are of special interest. Pre-existent OSA was present in 29% of cases, being higher than the 3.1% prevalence of OSA patients under treatment in our region. Because of this disproportionally high frequency of OSA, we performed a post-hoc analysis of those patients and their pre-existent condition. Even though obesity is by now an established risk factor for OSA and for severe Covid-19, weight alone does not explain the high proportion of patients with OSA, as obesity is a much more common pre-existing condition than OSA in the Finnish population with a prevalence of 26.1% among men and 27.5% among women.10 The high prevalence of pre-existing OSA in this cohort suggests that OSA, despite treatment, may be an independent risk factor for hospitalization. In fact, OSA was present in 21% of cases in a case series in Seattle and in 28.6% of critically ill Covid-19 patients in a cohort of Washington.11,12 Our finding of high OSA prevalence may help to understand pathogenesis of severe Covid-19.Several mechanisms may contribute to increased risk of severe Covid-19 in OSA patients, independent of overweight. Intermittent hypoxemia is one of the main pathologic features of OSA and could potentially worsen hypoxemia caused by Covid-19.13 OSA is also related to chronic inflammatory state and is associated with increased levels of proinflammatory markers such as ferritin, interleukin-6 and leptin, which may contribute to the risk for cytokine storm in Covid-19.13–15 Hypercoagulability is another pathophysiologic feature shared by both Covid-19 and OSA.16–18 Furthermore, RAAS may also be influenced by sleep apnea, and RAAS-associated regulation of ACE2-expression could be another mechanism by which OSA may contribute to the risk of severe Covid-19.7,8,19
A second striking observation is the high level of CRP in patients admitted to the ICU. Even though CRP has a well-established role in diagnostics and follow-up of bacterial pneumonia and other bacterial infections, the usefulness of CRP levels in viral infections has been less clear. Even though several studies were not able to distinguish viral pneumonia from pneumonia of bacterial origin based on CRP, CRP is often being used as guidance for treatment with antibiotics.20 Our data shows that high CRP correlates with native oxygen saturation and is associated with increased risk for ICU admission within several days. CRP levels may reflect the pulmonary damage caused by Covid-19.21 Theoretically, concomitant bacterial pneumonia can be present but undiagnosed. However, we found no evidence of bacterial infection, blood cultures remained negative in all patients and those with CRP>100 mg/L upon presentation had in most cases radiology findings consistent with Covid-19 without radiologic suspicion of bacterial pneumonia. Our study has some major limitations. First, the size of the study remains small due to low incidence of Covid-19 in Southwest Finland in the early phase of the pandemic. The question whether OSA is an independent risk factor should be addressed in larger cohorts. Second, OSA was not ruled out from the rest of the cohort and therefore the prevalence of OSA may be higher than reported. Third, due to the retrospective nature of this study, there was incomplete documentation on some of the variables. Fourth, follow-up was very short, which may lead to under reporting of mortality and complications, although the number still in hospital on the date of data cut-off was very low. Fifth, we only report on patients admitted in the hospital, which leads to an obvious bias, mild cases are largely missed, and fragile patients with severe disease may not be transported to the hospital but receive instead palliative care at home.
The high prevalence of pre-existing OSA in this cohort may have implications for individual risk assessment and may contribute to a better understanding of the pathogenesis of severe Covid-19. However, the finding should be considered with extreme caution, as this cohort is rather small. The question whether OSA is an independent risk factor should be addressed in larger cohorts. According to our findings, high CRP and PCT and possibly low native oxygen saturation on admission may be predictors for transfer to critical care. If confirmed in larger studies, this may be useful in triage which may be especially helpful when the epidemic is out of control and hospital places are scarce, requiring decision making based on basic clinical and laboratory assessment. The usability of CRP, PCT and SpO2 in clinical workup of Covid-19 has yet to be confirmed in larger studies.
We are grateful to Ton Feuth for his advices on the statistics used in this study.
The authors declare that there are no conflicts of interest.
None.

©2020 Feuth, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.