eISSN: 2576-4543


Review Article Volume 8 Issue 3
Physico-Technical Institute, A.F. Ioffe of the Russian Academy of Sciences, Russia
Correspondence: VA Ryzhov, Physico-Technical Institute, A.F. Ioffe of the Russian Academy of Sciences,194021 St. Petersburg, Russia
Received: June 23, 2024 | Published: July 15, 2024
Citation: Ryzhov VA. Features of the optical and structural properties of ionomers in the terahertz / far infrared range (˂ 300 CM-1). Phys Astron Int J. 2024;8(3):133‒136. DOI: 10.15406/paij.2024.08.00341
To study the optical and structural properties of ionomers, far infrared spectra of polystyrene sulfonic acid films containing alkali and alkaline earth metal ions were obtained and analyzed. Strong broad bands were found, the frequencies of which depend on cations, and they were assigned to the motion of cations in the Coulomb field of sulfonate groups. The frequencies of cation motion calculated for such a force field are close to the experimental frequencies. To study the effect of the formation of cation-anion associates of different organization in the system, polymer compositions based on sulfo-containing polyether urethane (PUI) and K+-ionomer of a styrene-acrylic acid copolymer (St-co-AA(K+)) were studied. It is shown that, depending on the ratio of components, both simple ion pairs and complex multiplets and clusters are formed in such systems.
Keywords: styrene-based Ionomers, sulfonic acid, multiplets and clusters, terahertz/far IR spectra, Coulomb interaction.
MB, Mitochondrial diseases; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; GTCS, generalized tonic-clonic seizure; HSV, herpes simplex virus; ENMG, electroneuromyography
Ion-containing copolymers - ionomers - are one of the most interesting objects of modern chemistry and physics of high-molecular compounds. Ionomers have higher elastic moduli in glass figurative state and strength than the corresponding non-ionized copolymers; they are characterized by increased thermal and electrical conductivity. Based on such compounds, new generations of materials and composites have already been created and continue to be developed for use in the technology of high-strength ion-exchange resins and polyelectrolytes, applications in holography, data storage systems, optoelectronics and optical filters.
Despite the general progress in the field of research on ionic copolymers, their structure and properties have not been systematically analyzed to date. This is all the more strange if we take into accounting the continued interest of scientists in metal-containing polymer systems in general. Relatively recently, it was found that the structural and rheological properties of these materials depend on the state of ion aggregation.1 However, most of the works concerned copolymers with an ionic comonomer concentration not exceeding 10–15%. It was found that for such compositions, the low dielectric constant of the main component promotes the formation of ion pairs and, through the formation of their multiplets, the formation of clusters.2
The possibility of using low-frequency IR spectra to study Coulomb (electrostatic) forces acting in ionomers and determining the presence of multiplets and clusters is shown in 3. Sensitivity of vibration frequency of cation-anion pairs to mutual the location of ions makes it possible to study the structure of ionic aggregates in polymer systems containing a small number of salt groups in the chain, when the use of other methods is ineffective. Dependence of the magnitude of cation-anion interaction on the degree of clustering, hydration and nature of the cation and the anionic part of the ionomer is shown in 4.
A feature of the spectra of these ionomers is the presence at terahertz frequencies of a wide, well-defined and intense absorption band, which is not present in the spectrum of the non-ionized copolymer. Since its position strongly depends on the mass of the cation, it can be attributed to the movement of the cation in the anion field of the copolymer. The large half-width of the band indicates that there are several options for the anionic environment of the cation, differing in the strength of Coulomb interactions. Another feature is that in ionomers containing an increased concentration of metal cations, additional absorption appears on the low-frequency wing of this band, indicating the formation of higher multiplets or clusters. Since in such ionic aggregates the interaction in the cation-anion cell is significantly screened by the environment, the vibration frequency of the cation is reduced compared to the vibration frequency in a simple multiplet.
This work continues the research begun in 5,6. The goal was to study, based on a more detailed analysis of the spectra, the optical properties of ionomers, which depend on the magnitude of the cation-anion interaction and the state of aggregation of the ions included in the polymer chain.
The interaction in the cation–anion cell, determined by the nature of the cation and the anionic part of the ionomers, was studied using spectra in the far IR region of ion-containing copolymers of styrene with salts of sulfonic acid (PSSA), the chemical composition of which can be represented as
- [СН2 - СН]n - [СН2 - С]m -
C6H5 SО3(-)М(+),
where M is a metal cation (Na, K, Rb, Cs, Ca, Sr and Ba), and the value m/m+n represents the molar fraction of carboxyl groups.
To study the influence of the formation of cation-anion associates of different organizational forms in the system, polymer compositions based on sulfonic polyether urethane (PUI) and the K+ ionomer of styrene copolymer with acrylic acid (St-co-AA(K+)), in which depending on the ratio of components, both simple ion pairs and complex multiplets and clusters are formed.
The synthesis of copolymers of styrene with sulfonic acid salts (PSSA) containing ions of alkali and alkaline earth metals, as well as the ionomer of linear sulfonic polyether urethane, PUI, was carried out at the Institute of Macromolecular Compound Chemistry of the National Academy of Sciences of Ukraine.6 St-co-AA(K+) was obtained by partial neutralization of the St-co-AA copolymer with a KOH solution. A random copolymer of styrene with acrylic acid, St-co-AA (St/AA = 45/55 mol. %), was obtained by radical copolymerization of St and AA in a block.
Film materials of the studied ionomers based on PSSC and PUI were obtained by evaporating the solvent at room temperature, followed by drying the films to constant weight in vacuum. Spectra in the far IR region (FIR) from 1.5 to 9 THz (50 – 300 cm-1) of films of copolymers and ionomers with a thickness of 0.1 - 1.0 mm were recorded on a Hitachi FIS-21 spectrometer at room temperature with a resolution of 1 - 2 cm-1. The position of the band maxima was determined with an error of 2 - 3 cm-1. The measurement error of the absorption coefficient k(ν) = ln(I0/I)/(t-t0), where I0 and I transmission of samples with thickness t and t0 ≈ 0.1t, respectively, was 10-15%.
In Figure 1 shows the far-IR spectra of styrene copolymers with sulfonic acid salts (PSSA) containing alkali and alkaline earth metal ions.
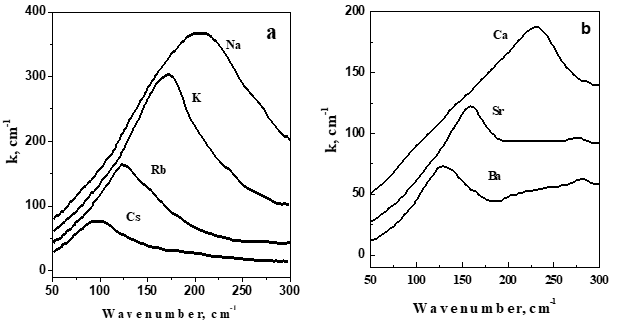
Figure 1 a) FIR spectra of Na+, K+, Rb+, Cs+ ionomers of PSSA; b) FIR spectra of Ca+, Sr+, Ba+ ionomers of PSSA.
In the range of 50-450 cm-1 of these spectra, the absorption bands associated with the movement of cations Na, K, Rb, Cs, Ca, Sr, Ba are the most intense. The position of the maximum of these bands vmax is clearly determined by the mass of the Mc cation, and the dependences of vmax on 1/ Mc for both alkali and alkaline earth ions are practically linear and differ from each other only in their slope (Figure 2a). Linearity means that the force constants of vibrations of ions belonging to the same group are close in value. And they are larger for alkaline earth ions, since the slope of the dependence of vmax on 1/ Mc is steeper here.
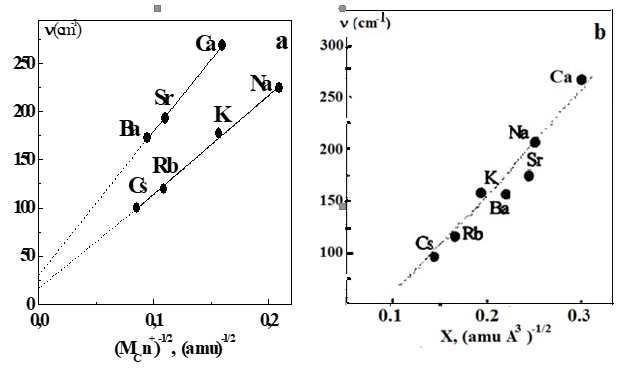
Figure 2 a) Graphs of the frequency of cation movement for various polysulfide ionomers; b) Dependence of parameter X on the vibration frequency of the cation in polysulfide ionomers.
Of course, identifying absorption bands associated with cation vibrations based solely on the fact that the frequency of these vibrations depends on the mass of the cation is not entirely correct. Firstly, because in the expression for the oscillator frequency v (cm-1) = (1/2πc)∙ (f/μ), where μ is the reduced mass, must take into account the immediate environment of the cation. Secondly, the force constant f = (d2U/dr2) at r = rmin, where U is the potential energy of interaction of ions, and r is the distance between them, will be determined by the nature of both the cation and the anion. The closest anionic environments of the cation in the studied PSSA ionomers are oxygen atoms in tetrahedral or octahedral geometry. Assuming an octahedral environment, we have μ = (Mc∙m0)/(2m0+Mc), where m0 is the mass of the oxygen atom.
The total interaction potential between a cation and anion can be represented as
U = Ucoulomb + Uinduc + Uvan der Waals + Uattract (1)
If the opposing forces between the cation and anion are predominantly Coulomb forces, then equation (1) can be written as:
U = qC• qA •e2/rCA + exp( –rCA/ρ), (2)
where qC is the charge of the cation, qA is the effective charge of the anion, e is the electrostatic charge, rCA is the cation-oxygen distance, ρ is a constant equal to 0.33 Å. The first term of this expression characterizes the Coulomb (electrostatic) attraction between opposite charges, the second - exchange repulsive forces.
The second derivative of the expression for the energy of interionic interactions (d2U/dr2) at r = rmin gives f = qC• qA ∙ e2 (r - 2ρ)/ πr3. With such f and μ, the vibration frequency of the cation is the position of the absorption band maximum –
vmax = [qA ∙ e2 / (2πc)2]1/2 ∙ [qC (r - 2ρ) / πμr3]1/2. (3)
Since for samples in which counter ions are present, the first part of equation 3 is a constant value, we obtain the following expression for determining vmax:
vmax = const•[ (qC (r - 2ρ)/πμr3]1/2 (4)
The obtained dependence for vmax can be represented in the form of a linear equation v = mX + b, in which the parameter X will be determined only by the nature of the cation, and the slope of the dependence m - by the effective charge of its anionic environment. The graph of v versus X is shown inFigure 2b.
It can be seen that the frequencies of the maxima of the cation vibration bands in the presented FIR spectra fall well on a straight line, confirming the assignment made. The validity of using the potential of ionic interactions allows us to have quantitative data on such (mainly Coulomb) interactions in ionomers. How Coulomb interactions change can be monitored in the FIR spectra of ionomers, for example, during dehydration, which is known to greatly affect the structural, mechanical and thermodynamic properties. In the FIR spectra of dehydrated ionomers, the cation vibration band shifts to low frequencies and noticeably narrows, which can be interpreted as a consequence of increased interactions between sulfate groups and the cation upon removal of molecularly adsorbed water. In the spectrum of the ionomer annealed at T>Tg, the vibration band of the cation also narrows, but the shift of its maximum cannot be detected. This result can be explained by the compensating effect of a decrease in the strength of Coulomb interactions with increasing temperature and the impossibility of forming ion clusters under these conditions. In light of the cluster mode, it is interesting to compare the spectra of ionomers with different concentrations of sulfate groups. As shown in 3, the vibration frequency of the cation actually decreases with increasing concentration of sulfate groups, since the vibration of the cation will be determined by both the increased effective mass and the reduced force constant due to the screening of cation-anion attraction.
In the FIR spectra of composites of sulfonic polyether urethane and K+ ionomer styrene copolymer with acrylic acid (ST-co-AA(K+)) we studied, the cation vibration band lies at 179±2 cm-1 (Figure 3). Calculation using formula (4) gives the value vmax = 180 ± 2 cm-1.
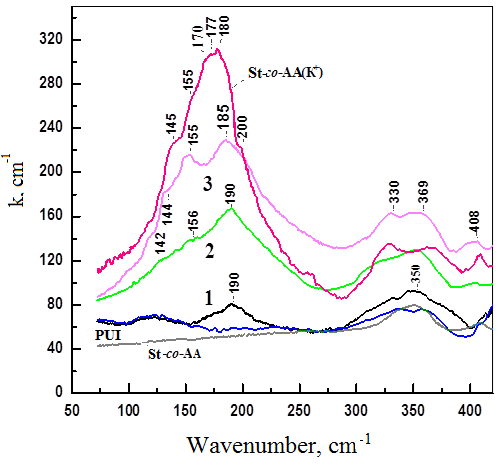
Figure 3 FIR spectra in the region 70 – 420 cm-1 of the original PUI, St-co-AA and St-co-AA(K+), as well as PUI/St-co-AA(K+) mixtures with different St contents -co-AA(K+) (wt.%): (1) 10; (2) 30 and (3) 50.
In Figure 3 shows the spectra of individual St-co-AA, St-co-AA(K+) and PUI, as well as mixtures of PUI/St-co-AA(K+) with different ratios of components. A distinctive feature of the spectrum of St-co-AA(K+) is the presence in the region below 300 cm-1 of a wide and clearly defined absorption band with a maximum around ν ~ 180 cm-1, which is not present in the spectra of St-co-AA and PUI. As was shown above, for K+ -containing copolymers of styrene with sulfonic acid, the presence of the indicated absorption band in this region of the IR spectrum is due to vibrations of K+ cations in the anionic environment of carboxylate (RCOO–) groups.
The significant half-width of this band is explained by the superposition of a number of overlapping absorption bands, the appearance of which reflects the presence of various types of cation-anion associates, from simple forms such as low-order ionic aggregates to complex forms of associates such as multiplets and clusters. Thus, the vibrations of K+ cations in low-order ionic aggregates are evidenced by the presence of an absorption band with a maximum at ν ~ 200 cm-1, and the vibrations of K+ cations in cation-anion associates (multiplets) consisting of several ion pairs are evidenced by bands with maxima at ν ~ 177 and ν ~ 170 cm-1. That is, the greater the shift of the absorption band towards lower frequencies, the larger the size of the ionic aggregates in which the K+ cations perform their vibrational movements. Therefore, the presence of absorption bands at ν ~ 155 and ν ~ 145 cm-1 in the spectrum may be associated with vibrations of K+ cations in clusters formed by the association of a large number of multiplets.
In 4, models of the most probable arrangement of the K+ cation and carboxylate groups are discussed, in which the existence of various vibrational associates is assumed. Thus, in model 1, an infinitely large environment of the cation is assumed by oxygen atoms of carboxylate groups, in model 2, the metal ion is associated with only one carboxylate group, in model 3 – with two, and model 4 assumes an octahedral environment of the K+ cation with oxygen of RCOO─ groups. It is shown that the strongest interaction between the cation and the anion will be observed in model 1, which assumes an infinitely large environment of the cation by oxygen atoms of carboxylate groups, and the weakest interaction will be observed in model 4, where an octahedral environment of the K+ cation with oxygen of RCOO─ groups is assumed.
Using the method of Gaussian distribution of curves, it is possible to calculate the content of ionic aggregates (clusters and multiplets) and low-order ionic aggregates in the samples under study depending on their composition. This calculation shows that in a sample with the composition PUI/St-co-AA(K+ = 90/10), K+ cations participate in the formation of only low-order aggregates and multiplets. In samples with a higher content of St-co-AA(K+) (> 30%) K+ cations take part in the formation of both low-order ionic aggregates and multiplets, and ionic clusters, the content of which increases with increasing proportion of St-co-AA(K+).
Data on the relative content of ionic aggregates (clusters and multiplets) and associates (low-order ionic aggregates) in PUI/St-co-AA(K+) mixtures with different contents of St-co-AA(K+) are presented in Figure 4.
From the data presented in Figure 4, it follows that during the formation of the micro phase structure of mixtures with a small content (up to ~ 20 wt. %) St-co-AA(K+), low-order ionic aggregates are predominantly formed, and their content is higher than could be expected assume based on the additive contribution of the components. In mixtures containing > 30 wt. % St-co-AA(K+), the formation of ionic clusters predominates, and the proportion of multiplets and low-order ionic aggregates decreases, especially at a content of 50 wt.% St-co-AA(K+). It is interesting to note that in mixtures of the indicated compositions the content of low-order ionic aggregates and associates is higher than it should be based on the rule of additive contribution of components. It can be assumed that in all the studied samples, the formation of both intra- and inter-molecular (between components) ionic bonds of various types takes place.
So, firstly, the far-infrared spectra of Na, K, Rb, Cs, Ca, Sr and Ba salts of styrene copolymers with sulfonic acid in the region of 300–33 cm–1 at room temperature were studied here. The broad band, which shifts toward lower frequencies as the mass of the cation increases, was attributed to the motion of cations in the Coulomb field of sulfonate groups, since the frequencies of motion of cations calculated for such a force field are close to the frequencies of the maxima of these bands. The validity of using the potential of ionic interactions allows us to have quantitative data on such (mainly Coulomb) interactions in ionomers and, using the FIR spectra of ionomers, to monitor how Coulomb interactions change, for example, during dehydration, which, as is known, greatly affects the structural, mechanical and thermodynamic properties.
Secondly, far-field IR spectroscopy data convincingly indicate the presence of a network of ionic bonds, as well as supramolecular ionic aggregates (clusters and multiplets) in St-co-AA(K+) - a component of PUI/St-co-AA(K +) compositions. It has been established that during the formation of the micro phase structure of mixtures with a small content of St-co-AA(K+), the destruction of ionic aggregates (clusters and multiplets) and the intensive formation of low-order ionic aggregates occur. With an increase in the proportion of St-co-AA(K+) in the system, the formation of ionic clusters begins to predominate. The latter may indicate an increase in micro phase separation of components in the mixtures under study. We believe that in all the studied samples, the formation of both intra- and inter-molecular (between components) ionic bonds of various types takes place.
The presented results of studying the optical and structural properties of ion-containing copolymers of styrene with sulfonic acid salts (PSSA) show the promise of low-frequency IR spectroscopy for obtaining information about Coulomb interactions and the microstructure of ionomers.

©2024 Ryzhov. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.